Abstract
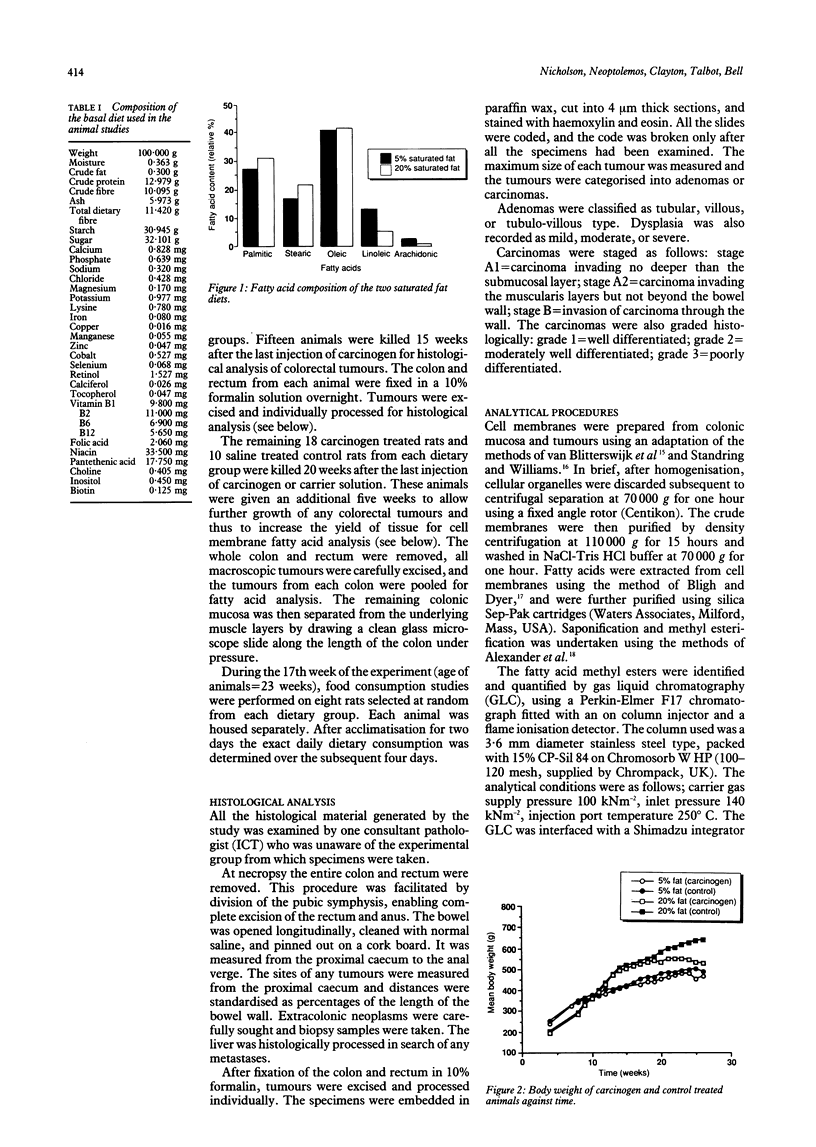
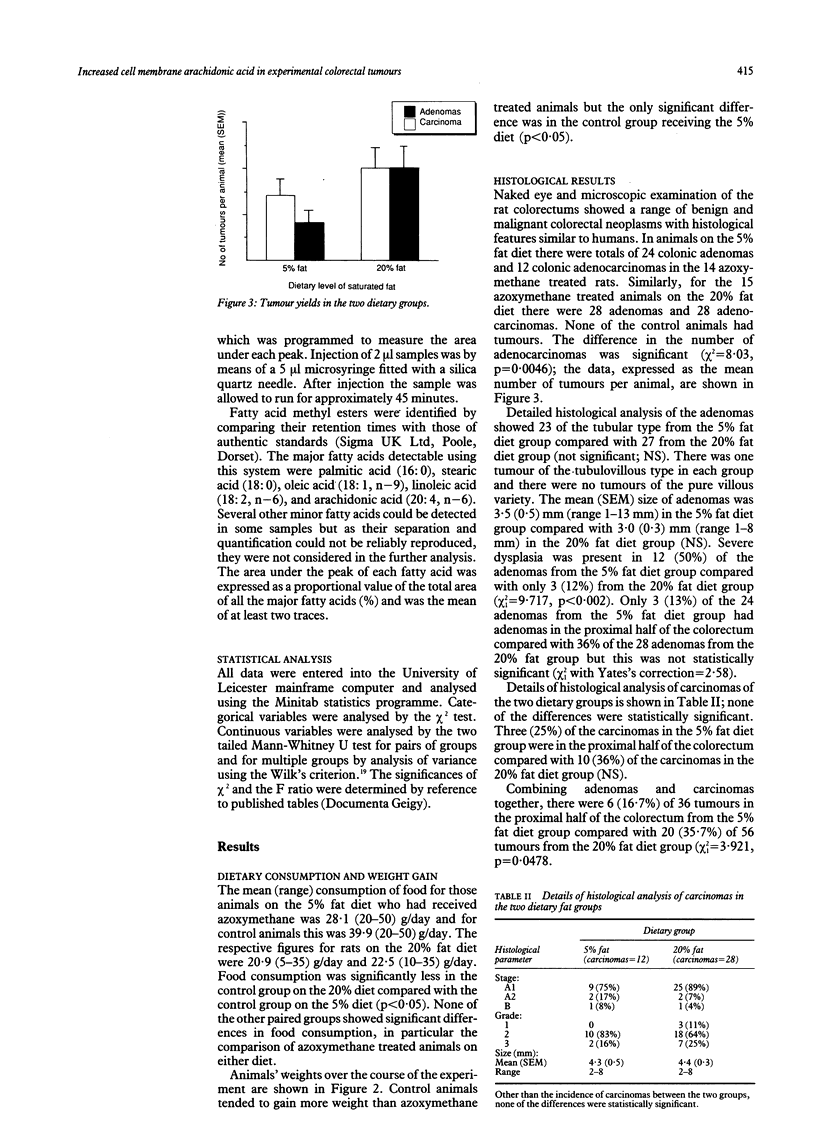
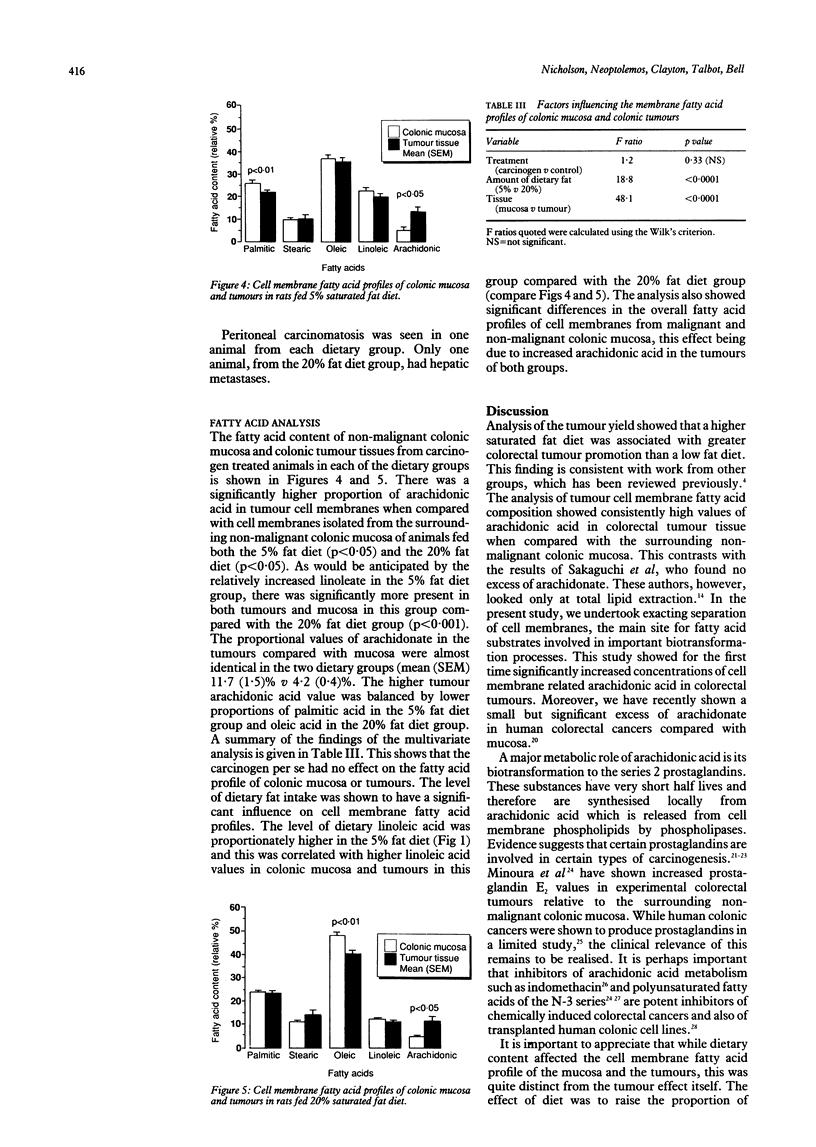
Tumour cell membrane fatty acid composition was investigated using an animal model of colorectal carcinogenesis. Eighty six male Wistar rats were fed experimental diets containing either 5% saturated fat or 20% saturated fat. Colorectal tumours were induced by intraperitoneal injection of azoxymethane, and control rats received saline. Animals were killed at intervals up to 26 weeks after the last injection of carcinogen for histology and lipid analysis. Cell membrane fatty acids in colonic mucosa and colorectal tumours were determined by gas liquid chromatography. Animals fed the 20% fat diet developed more carcinomas (28 cancers in 14 rats) than those fed the 5% fat diet (14 cancers in 15 rats; chi 2 = 8.03, p = 0.0046) but they did not develop significantly more adenomas (28 and 24 respectively). Cell membrane fatty acid analysis showed a considerable increase in the content of arachidonic acid (20:4, n-6) in the tumours (mean (SEM) 11.7 (1.5)%) compared with colonic mucosa (4.2 (0.4)%; p less than 0.05). Dietary fatty acid composition was also found to influence the profile of fatty acids in the colonic mucosa. This study suggests that a high saturated fat diet promotes the malignant transformation of colorectal adenomas. The colorectal tumours were characterised by an increased cell membrane arachidonic acid, the precursor of putative cancer promoting prostaglandins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander L. R., Justice J. B., Jr, Madden J. Fatty acid composition of human erythrocyte membranes by capillary gas chromatography-mass spectrometry. J Chromatogr. 1985 Jul 12;342(1):1–12. doi: 10.1016/s0378-4347(00)84484-9. [DOI] [PubMed] [Google Scholar]
- Armstrong B., Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975 Apr 15;15(4):617–631. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- Bennett A., Tacca M. D., Stamford I. F., Zebro T. Prostaglandins from tumours of human large bowel. Br J Cancer. 1977 Jun;35(6):881–884. doi: 10.1038/bjc.1977.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas R., Basu M., Sen-Majumdar A., Das M. Intrapeptide autophosphorylation of the epidermal growth factor receptor: regulation of kinase catalytic function by receptor dimerization. Biochemistry. 1985 Jul 2;24(14):3795–3802. doi: 10.1021/bi00335a056. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Davidson N. O., Schachter D. Variations in dietary triacylglycerol saturation alter the lipid composition and fluidity of rat intestinal plasma membranes. Biochim Biophys Acta. 1985 Jan 25;812(2):460–472. doi: 10.1016/0005-2736(85)90321-9. [DOI] [PubMed] [Google Scholar]
- Bull A. W., Soullier B. K., Wilson P. S., Hayden M. T., Nigro N. D. Promotion of azoxymethane-induced intestinal cancer by high-fat diet in rats. Cancer Res. 1979 Dec;39(12):4956–4959. [PubMed] [Google Scholar]
- Burns C. P., North J. A. Adriamycin transport and sensitivity in fatty acid-modified leukemia cells. Biochim Biophys Acta. 1986 Aug 29;888(1):10–17. doi: 10.1016/0167-4889(86)90064-9. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983 Oct;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Haeffner E. W., Heck B., Kolbe K. Difference in plasma membrane structure between two sublines of Ehrlich-Lettrè ascites tumor cells. Biochim Biophys Acta. 1982 Dec 22;693(2):280–286. doi: 10.1016/0005-2736(82)90433-3. [DOI] [PubMed] [Google Scholar]
- Horwitz A. F., Hatten M. E., Burger M. M. Membrane fatty acid replacements and their effect on growth and lectin-induced agglutinability. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3115–3119. doi: 10.1073/pnas.71.8.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffcoat R., Roberts P. A., James A. T. The control of lipogenesis by dietary linoleic acid and its influence on the deposition of fat. Eur J Biochem. 1979 Nov;101(2):447–453. doi: 10.1111/j.1432-1033.1979.tb19738.x. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D., Weber M. M., Klurfeld D. M. Dietary fat versus caloric content in initiation and promotion of 7,12-dimethylbenz(a)anthracene-induced mammary tumorigenesis in rats. Cancer Res. 1984 Aug;44(8):3174–3177. [PubMed] [Google Scholar]
- Lynch N. R., Castes M., Astoin M., Salomon J. C. Mechanism of inhibition of tumour growth by aspirin and indomethacin. Br J Cancer. 1978 Oct;38(4):503–512. doi: 10.1038/bjc.1978.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoura T., Takata T., Sakaguchi M., Takada H., Yamamura M., Hioki K., Yamamoto M. Effect of dietary eicosapentaenoic acid on azoxymethane-induced colon carcinogenesis in rats. Cancer Res. 1988 Sep 1;48(17):4790–4794. [PubMed] [Google Scholar]
- Morson B. C. Evolution of cancer of the colon and rectum. Cancer. 1974 Sep;34(3):suppl–suppl:849. doi: 10.1002/1097-0142(197409)34:3+<845::aid-cncr2820340710>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Narisawa T., Sato M., Sano M., Niwa M., Takahashi M., Ito T., Tanida N., Shimoyama T. Prevention of colon polyposis and carcinomas by right hemicolectomy and indomethacin in animal model. Cancer. 1985 Oct 1;56(7):1719–1724. doi: 10.1002/1097-0142(19851001)56:7<1719::aid-cncr2820560742>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Neoptolemos J. P., Clayton H., Heagerty A. M., Nicholson M. J., Johnson B., Mason J., Manson K., James R. F., Bell P. R. Dietary fat in relation to fatty acid composition of red cells and adipose tissue in colorectal cancer. Br J Cancer. 1988 Nov;58(5):575–579. doi: 10.1038/bjc.1988.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro N. D., Singh D. V., Campbell R. L., Sook M. Effect of dietary beef fat on intestinal tumor formation by azoxymethane in rats. J Natl Cancer Inst. 1975 Feb;54(2):439–442. [PubMed] [Google Scholar]
- Pollard M., Luckert P. H. Effect of indomethacin on intestinal tumors induced in rats by the acetate derivative of dimethylnitrosamine. Science. 1981 Oct 30;214(4520):558–559. doi: 10.1126/science.7291992. [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Maeura Y. Tumor promotion by dietary fat in azoxymethane-induced colon carcinogenesis in female F344 rats: influence of amount and source of dietary fat. J Natl Cancer Inst. 1984 Mar;72(3):745–750. [PubMed] [Google Scholar]
- Reddy B. S., Narisawa T., Maronpot R., Weisburger J. H., Wynder E. L. Animal models for the study of dietary factors and cancer of the large bowel. Cancer Res. 1975 Nov;35(11 Pt 2):3421–3426. [PubMed] [Google Scholar]
- Reddy B. S., Sugie S. Effect of different levels of omega-3 and omega-6 fatty acids on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Res. 1988 Dec 1;48(23):6642–6647. [PubMed] [Google Scholar]
- Reddy B. S., Watanabe K., Weisburger J. H. Effect of high-fat diet on colon carcinogenesis in F344 rats treated with 1,2-dimethylhydrazine, methylazoxymethanol acetate, or methylnitrosourea. Cancer Res. 1977 Nov;37(11):4156–4159. [PubMed] [Google Scholar]
- Sakaguchi M., Hiramatsu Y., Takada H., Yamamura M., Hioki K., Saito K., Yamamoto M. Effect of dietary unsaturated and saturated fats on azoxymethane-induced colon carcinogenesis in rats. Cancer Res. 1984 Apr;44(4):1472–1477. [PubMed] [Google Scholar]
- Sakaguchi M., Minoura T., Hiramatsu Y., Takada H., Yamamura M., Hioki K., Yamamoto M. Effects of dietary saturated and unsaturated fatty acids on fecal bile acids and colon carcinogenesis induced by azoxymethane in rats. Cancer Res. 1986 Jan;46(1):61–65. [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Rivnay B. Degree of exposure of membrane proteins determined by fluorescence quenching. Biochemistry. 1977 Mar 8;16(5):982–986. doi: 10.1021/bi00624a027. [DOI] [PubMed] [Google Scholar]
- Smedley-Maclean I., Nunn L. C. Fat-deficiency disease of rats. The relation of the essential unsaturated acids to tumour formation in the albino rat on normal diet. Biochem J. 1941 Sep;35(8-9):983–989. doi: 10.1042/bj0350983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring R., Williams A. F. Glycoproteins and antigens of membranes prepared from rat thymocytes after lysis by shearing or with the detergent Tween-40. Biochim Biophys Acta. 1978 Mar 21;508(1):85–96. doi: 10.1016/0005-2736(78)90190-6. [DOI] [PubMed] [Google Scholar]
- Stubbs C. D., Smith A. D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984 Jan 27;779(1):89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Vitols S., Gahrton G., Björkholm M., Peterson C. Hypocholesterolaemia in malignancy due to elevated low-density-lipoprotein-receptor activity in tumour cells: evidence from studies in patients with leukaemia. Lancet. 1985 Nov 23;2(8465):1150–1154. doi: 10.1016/s0140-6736(85)92679-0. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk W. J., Emmelot P., Feltkamp C. A. Studies on plasma membranes. XIX. Isolation and characterization of a plasma membrane fraction from calf thymocytes. Biochim Biophys Acta. 1973 Mar 29;298(3):577–592. doi: 10.1016/0005-2736(73)90075-8. [DOI] [PubMed] [Google Scholar]


