Abstract
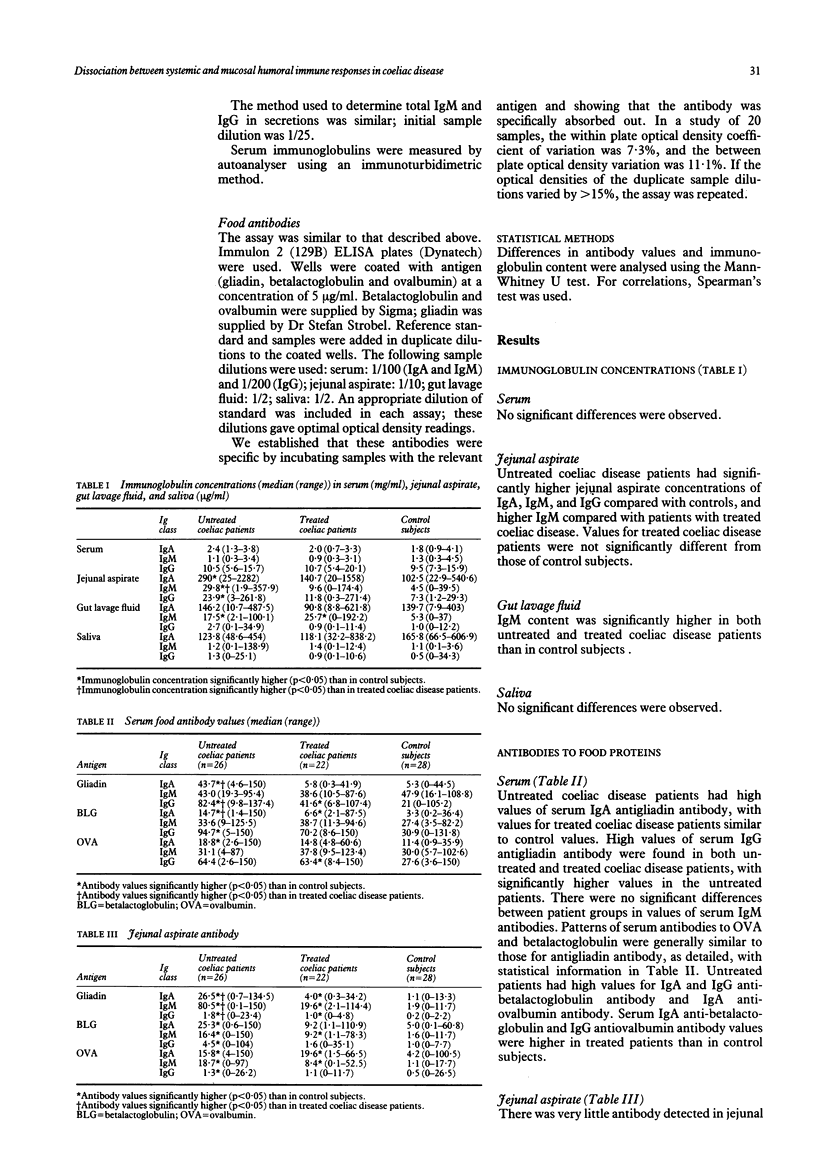
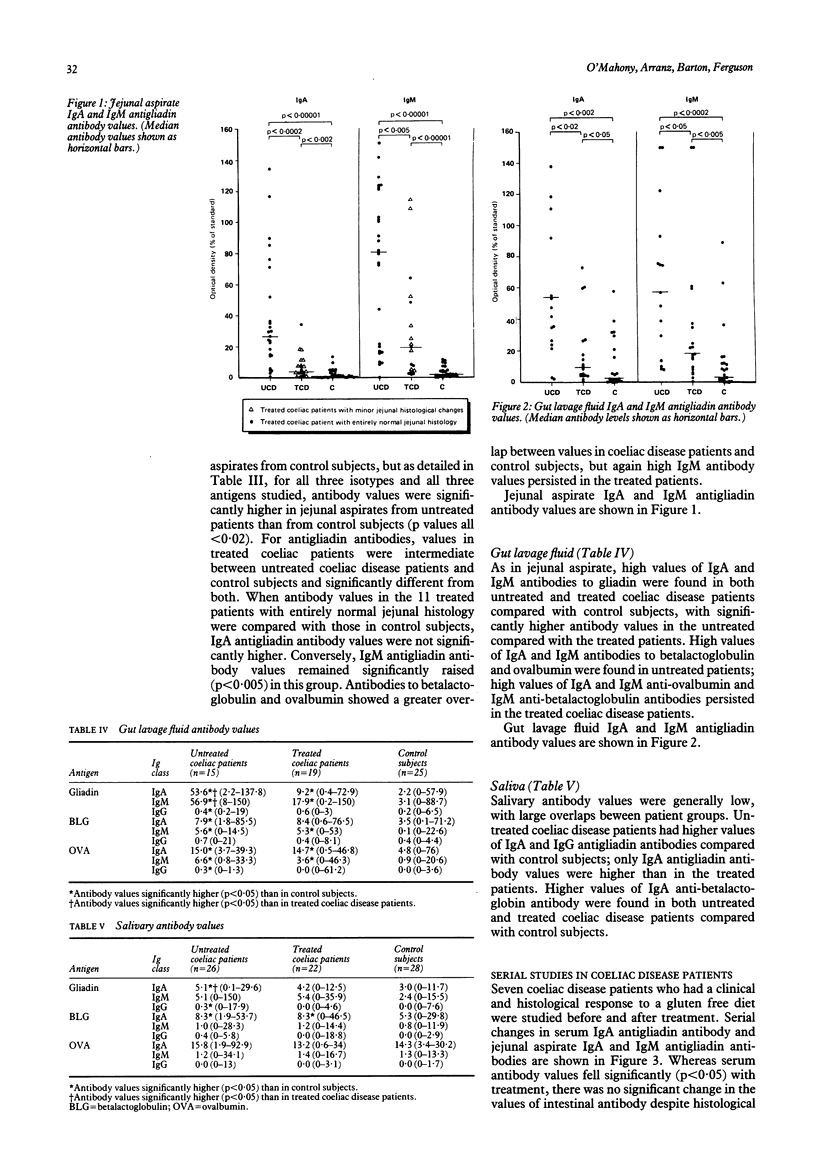
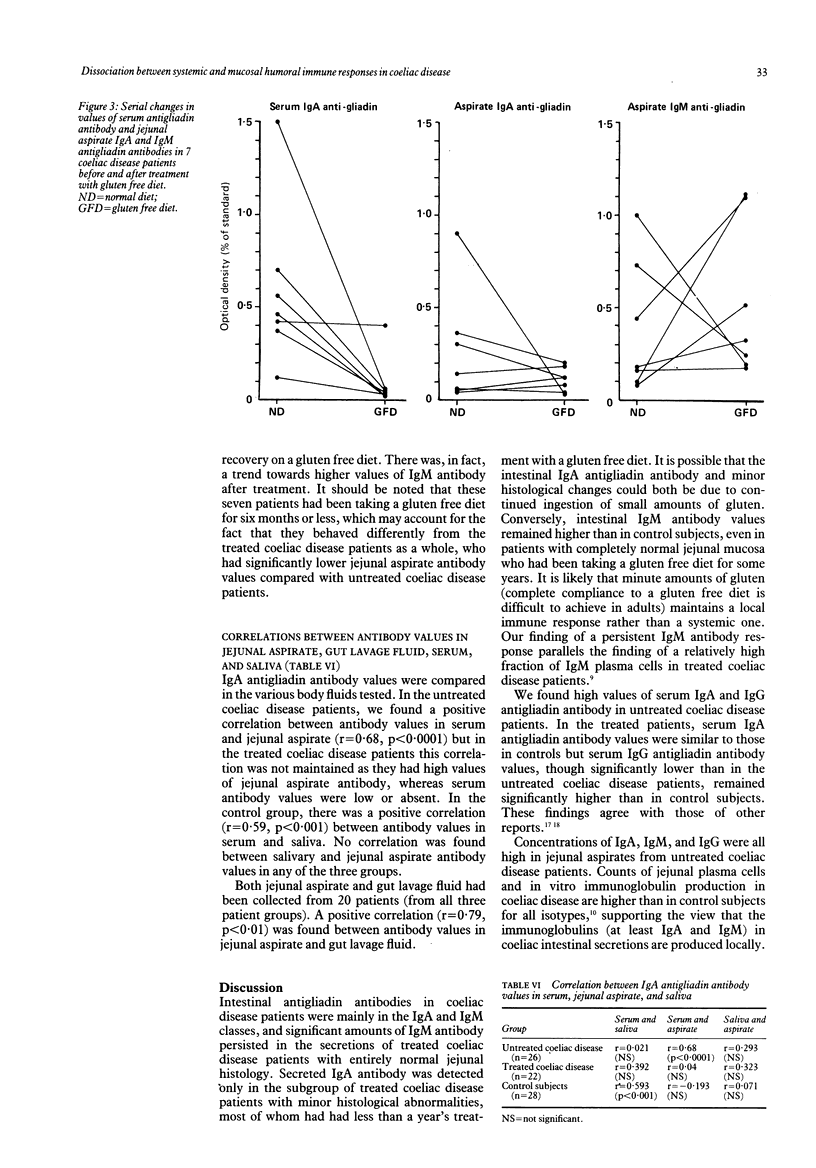
We examined humoral immunity in coeliac disease as expressed in serum (systemic immunity), and in saliva, jejunal aspirate, and whole gut lavage fluid (mucosal immunity). The aims were to define features of the secretory immune response (IgA and IgM concentrations and antibody values to gliadin and other food proteins measured by enzyme linked immunosorbent assay (ELISA)) in active disease and remission, and to establish whether secretions obtained by relatively non-invasive techniques (saliva and gut lavage fluid) can be used for indirect measurements of events in the jejunum. Serum, saliva, and jejunal aspirate from 26 adults with untreated coeliac disease, 22 treated patients, and 28 immunologically normal control subjects were studied, together with intestinal secretions obtained by gut lavage from 15 untreated and 19 treated patients with coeliac disease and 25 control subjects. Jejunal aspirate IgA and IgM and gut lavage fluid IgM concentrations were significantly raised in patients with untreated coeliac disease; the lavage fluid IgM concentration remained higher in patients with treated coeliac disease than in controls. Serum and salivary immunoglobulin concentrations were similar in the three groups. Patients with untreated coeliac disease had higher values of antibodies to gliadin compared with treated patients and control subjects in all body fluids tested; these were predominantly of IgA and IgG classes in serum, and of IgA and IgM classes in jejunal aspirate and gut lavage fluid. Values of salivary IgA antibodies to gliadin were significantly higher in untreated coeliacs, though antibody values were generally low, with a large overlap between coeliac disease patients and control subjects. In treated patients, with proved histological recovery on gluten free diet, serum IgA antigliadin antibody values fell to control values, though serum IgG antigliadin antibody values remained moderately raised. In contrast, there was persistence of secretory antigliadin antibodies in treated patients (particularly IgM antibody) in both jejunal aspirate and gut lavage fluid. Antibody responses to betalactoglobulin and ovalbumin were similar to those for gliadin, including persistence of high intestinal antibody values in patients with treated coeliac disease. There was a positive correlation between antibody values in jejunal aspirate and gut lavage fluid, but not between saliva and jejunal aspirate; thus salivary antibodies do not reflect intestinal humoral immunity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baklien K., Brandtzaeg P., Fausa O. Immunoglobulins in jejunal mucosa and serum from patients with adult coeliac disease. Scand J Gastroenterol. 1977;12(2):149–159. [PubMed] [Google Scholar]
- Bullen A. W., Losowsky M. S. Cell-mediated immunity to gluten fraction III in adult coeliac disease. Gut. 1978 Feb;19(2):126–131. doi: 10.1136/gut.19.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciclitira P. J., Ellis H. J., Wood G. M., Howdle P. D., Losowsky M. S. Secretion of gliadin antibody by coeliac jejunal mucosal biopsies cultured in vitro. Clin Exp Immunol. 1986 Apr;64(1):119–124. [PMC free article] [PubMed] [Google Scholar]
- Falchuk Z. M., Strober W. Gluten-sensitive enteropathy: synthesis of antigliadin antibody in vitro. Gut. 1974 Dec;15(12):947–952. doi: 10.1136/gut.15.12.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A., Carswell F. Precipitins to dietary proteins in serum and upper intestinal secretions of coeliac children. Br Med J. 1972 Jan 8;1(5792):75–77. doi: 10.1136/bmj.1.5792.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A., MacDonald T. T., McClure J. P., Holden R. J. Cell-mediated immunity to gliadin within the small-intestinal mucosa in coeliac disease. Lancet. 1975 Apr 19;1(7912):895–897. doi: 10.1016/s0140-6736(75)91689-x. [DOI] [PubMed] [Google Scholar]
- Gaspari M. M., Brennan P. T., Solomon S. M., Elson C. O. A method of obtaining, processing, and analyzing human intestinal secretions for antibody content. J Immunol Methods. 1988 May 25;110(1):85–91. doi: 10.1016/0022-1759(88)90086-5. [DOI] [PubMed] [Google Scholar]
- Hohmann A., LaBrooy J., Davidson G. P., Shearman D. J. Measurement of specific antibodies in human intestinal aspirate: effect of the protease inhibitor phenylmethylsulphonyl fluoride. J Immunol Methods. 1983 Nov 11;64(1-2):199–204. doi: 10.1016/0022-1759(83)90398-8. [DOI] [PubMed] [Google Scholar]
- Kilander A. F., Nilsson L. A., Gillberg R. Serum antibodies to gliadin in coeliac disease after gluten withdrawal. Scand J Gastroenterol. 1987 Jan;22(1):29–34. doi: 10.3109/00365528708991852. [DOI] [PubMed] [Google Scholar]
- Labrooy J. T., Hohmann A. W., Davidson G. P., Hetzel P. A., Johnson R. B., Shearman D. J. Intestinal and serum antibody in coeliac disease: a comparison using ELISA. Clin Exp Immunol. 1986 Dec;66(3):661–668. [PMC free article] [PubMed] [Google Scholar]
- Marsh M. N. Immunocytes, enterocytes and the lamina propria: an immunopathological framework of coeliac disease. J R Coll Physicians Lond. 1983 Oct;17(4):205–212. [PMC free article] [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987 Jul;7(4):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- O'Farrelly C., Kelly J., Hekkens W., Bradley B., Thompson A., Feighery C., Weir D. G. Alpha gliadin antibody levels: a serological test for coeliac disease. Br Med J (Clin Res Ed) 1983 Jun 25;286(6383):2007–2010. doi: 10.1136/bmj.286.6383.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savilahti E., Viander M., Perkkiö M., Vainio E., Kalimo K., Reunala T. IgA antigliadin antibodies: a marker of mucosal damage in childhood coeliac disease. Lancet. 1983 Feb 12;1(8320):320–322. doi: 10.1016/s0140-6736(83)91627-6. [DOI] [PubMed] [Google Scholar]
- Scott H., Ek J., Brandtzaeg P. Changes of serum antibody activities to various dietary antigens related to gluten withdrawal or challenge in children with coeliac disease. Int Arch Allergy Appl Immunol. 1985;76(2):138–144. doi: 10.1159/000233680. [DOI] [PubMed] [Google Scholar]
- Scott H., Fausa O., Ek J., Brandtzaeg P. Immune response patterns in coeliac disease. Serum antibodies to dietary antigens measured by an enzyme linked immunosorbent assay (ELISA). Clin Exp Immunol. 1984 Jul;57(1):25–32. [PMC free article] [PubMed] [Google Scholar]
- Skerritt J. H., Johnson R. B., Hetzel P. A., La Brooy J. T., Shearman D. J., Davidson G. P. Variation of serum and intestinal gluten antibody specificities in coeliac disease. Clin Exp Immunol. 1987 Apr;68(1):189–199. [PMC free article] [PubMed] [Google Scholar]
- Unsworth D. J., Kieffer M., Holborow E. J., Coombs R. R., Walker-Smith J. A. IgA anti-gliadin antibodies in coeliac disease. Clin Exp Immunol. 1981 Nov;46(2):286–293. [PMC free article] [PubMed] [Google Scholar]
- Wood G. M., Howdle P. D., Trejdosiewicz L. K., Losowsky M. S. Jejunal plasma cells and in vitro immunoglobulin production in adult coeliac disease. Clin Exp Immunol. 1987 Jul;69(1):123–132. [PMC free article] [PubMed] [Google Scholar]


