Abstract
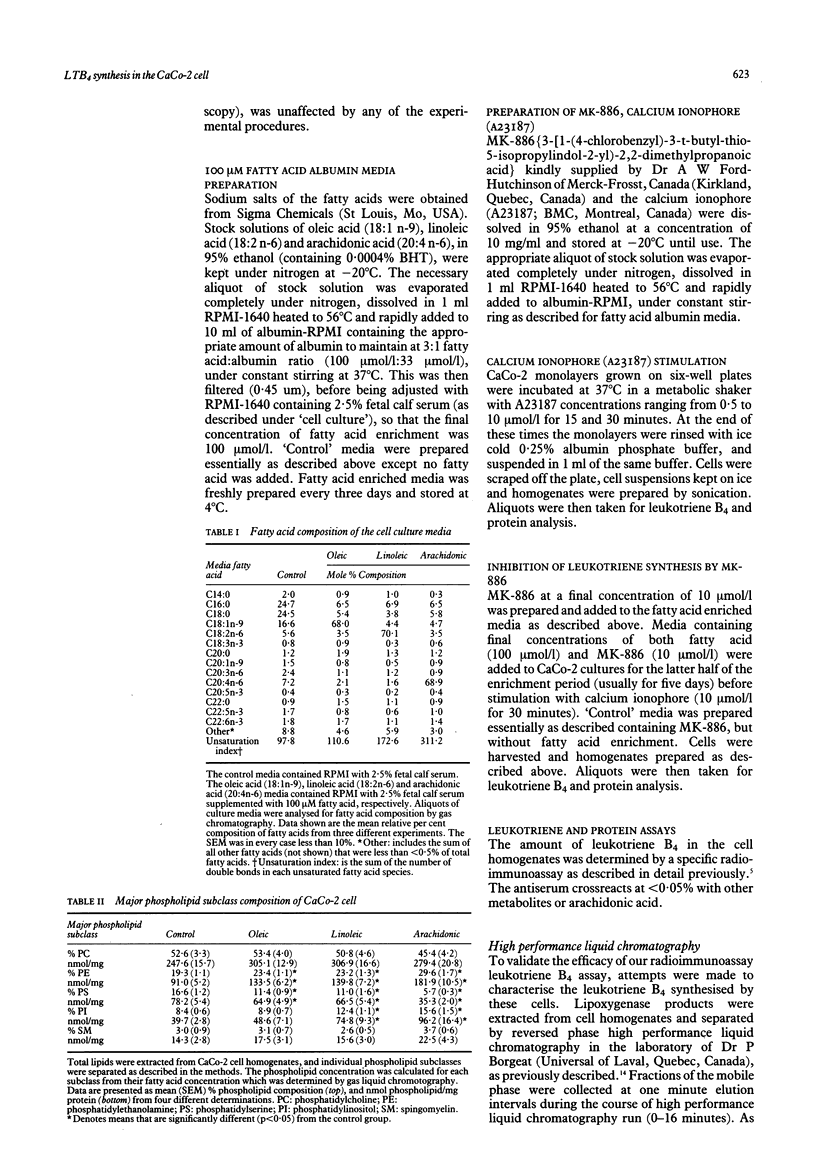
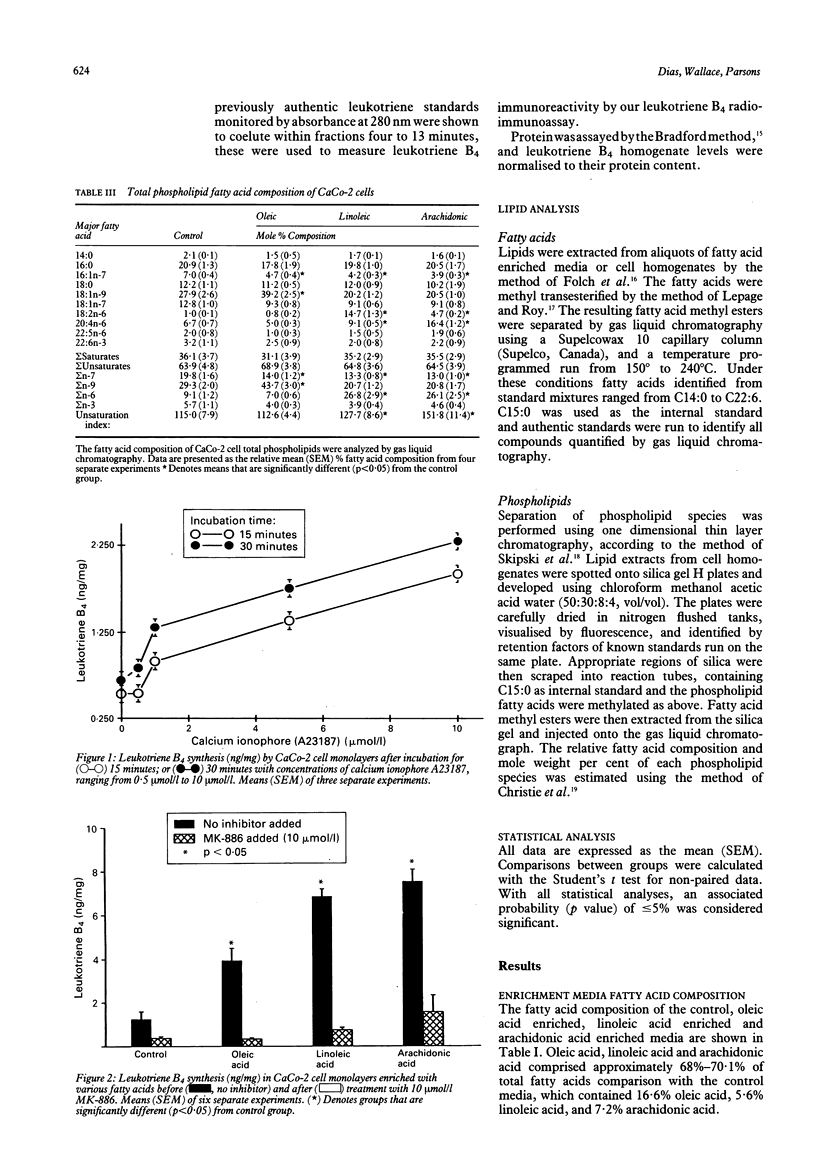
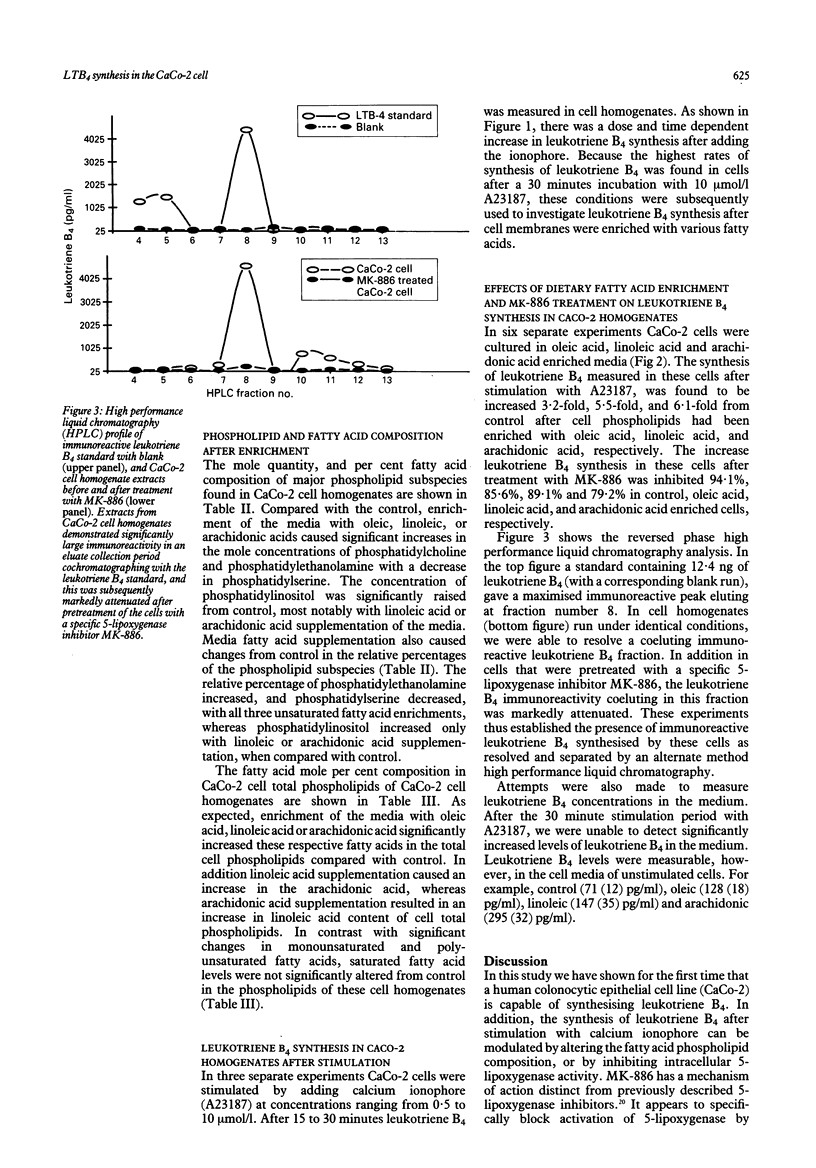
The ability of a human colonocyte epithelial cell line (CaCo-2) to synthesise leukotriene B4 was examined. In addition, the effects of stimulation with calcium ionophore, inhibition by a drug which specifically prevents activation of 5-lipoxygenase, and modification of the fatty acid composition of membrane phospholipids on leukotriene B4 synthesis were assessed. Incubation with calcium ionophore (A23187) resulted in a dose and time dependent increase in leukotriene B4 synthesis. After cell phospholipids had been enriched with oleic acid, linoleic acid, and arachidonic acid, leukotriene B4 synthesis was found to be increased 3.2-fold, 5.5-fold, and 6.1-fold above control. Treatment with MK-886 inhibited leukotriene B4 synthesis by 79% to 94% in the various groups. Variations in the polyunsaturated fatty acid content of intestinal epithelial cells could be important in the modulation of cellular responses. We have shown for the first time in this human intestinal epithelial cell its ability to synthesise leukotriene B4. In addition, leukotriene B4 synthesis can be modulated by the fatty acid composition of membrane phospholipids, which can be altered by dietary fatty acids. The synthesis of chemotatic factors, such as leukotriene B4, by the mucosal epithelium may contribute to the recruitment of granulocytes into the colonic mucosa and across the epithelium, giving rise to the crypt abscesses which characterise ulcerative colitis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgeat P., Picard S., Vallerand P., Bourgoin S., Odeimat A., Sirois P., Poubelle P. E. Automated on-line extraction and profiling of lipoxygenase products of arachidonic acid by high-performance liquid chromatography. Methods Enzymol. 1990;187:98–116. doi: 10.1016/0076-6879(90)87014-t. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chantret I., Barbat A., Dussaulx E., Brattain M. G., Zweibaum A. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res. 1988 Apr 1;48(7):1936–1942. [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Profiles of eicosanoid production by superficial and proliferative colonic epithelial cells and sub-epithelial colonic tissue. Prostaglandins. 1986 Sep;32(3):387–399. doi: 10.1016/0090-6980(86)90007-9. [DOI] [PubMed] [Google Scholar]
- Dashti N., Smith E. A., Alaupovic P. Increased production of apolipoprotein B and its lipoproteins by oleic acid in Caco-2 cells. J Lipid Res. 1990 Jan;31(1):113–123. [PubMed] [Google Scholar]
- Dreyling K. W., Hoppe U., Peskar B. A., Morgenroth K., Kozuschek W., Peskar B. M. Leukotriene synthesis by human gastrointestinal tissues. Biochim Biophys Acta. 1986 Sep 12;878(2):184–193. doi: 10.1016/0005-2760(86)90145-1. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Garg M. L., Thomson A. B., Clandinin M. T. Interactions of saturated, n-6 and n-3 polyunsaturated fatty acids to modulate arachidonic acid metabolism. J Lipid Res. 1990 Feb;31(2):271–277. [PubMed] [Google Scholar]
- Gillard J., Ford-Hutchinson A. W., Chan C., Charleson S., Denis D., Foster A., Fortin R., Leger S., McFarlane C. S., Morton H. L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2 - dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can J Physiol Pharmacol. 1989 May;67(5):456–464. doi: 10.1139/y89-073. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Granger D. N. 5-Aminosalicylic acid concentration in mucosal interstitium of cat small and large intestine. Dig Dis Sci. 1989 Apr;34(4):573–578. doi: 10.1007/BF01536335. [DOI] [PubMed] [Google Scholar]
- Hidalgo I. J., Raub T. J., Borchardt R. T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989 Mar;96(3):736–749. [PubMed] [Google Scholar]
- Lauritsen K., Laursen L. S., Bukhave K., Rask-Madsen J. Effects of topical 5-aminosalicylic acid and prednisolone on prostaglandin E2 and leukotriene B4 levels determined by equilibrium in vivo dialysis of rectum in relapsing ulcerative colitis. Gastroenterology. 1986 Oct;91(4):837–844. doi: 10.1016/0016-5085(86)90684-0. [DOI] [PubMed] [Google Scholar]
- Laursen L. S., Naesdal J., Bukhave K., Lauritsen K., Rask-Madsen J. Selective 5-lipoxygenase inhibition in ulcerative colitis. Lancet. 1990 Mar 24;335(8691):683–685. doi: 10.1016/0140-6736(90)90803-d. [DOI] [PubMed] [Google Scholar]
- Lepage G., Roy C. C. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986 Jan;27(1):114–120. [PubMed] [Google Scholar]
- Lobos E. A., Sharon P., Stenson W. F. Chemotactic activity in inflammatory bowel disease. Role of leukotriene B4. Dig Dis Sci. 1987 Dec;32(12):1380–1388. doi: 10.1007/BF01296664. [DOI] [PubMed] [Google Scholar]
- Murthy S., Albright E., Mathur S. N., Field F. J. Modification of CaCo-2 cell membrane fatty acid composition by eicosapentaenoic acid and palmitic acid: effect on cholesterol metabolism. J Lipid Res. 1988 Jun;29(6):773–780. [PubMed] [Google Scholar]
- Riehl T. E., Bass N. M., Stenson W. F. Metabolism of 15-hydroxyeicosatetraenoic acid by Caco-2 cells. J Lipid Res. 1990 May;31(5):773–780. [PubMed] [Google Scholar]
- Rouzer C. A., Ford-Hutchinson A. W., Morton H. E., Gillard J. W. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J Biol Chem. 1990 Jan 25;265(3):1436–1442. [PubMed] [Google Scholar]
- Spector A. A., Yorek M. A. Membrane lipid composition and cellular function. J Lipid Res. 1985 Sep;26(9):1015–1035. [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M. An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol. 1990 Apr;258(4 Pt 1):G527–G534. doi: 10.1152/ajpgi.1990.258.4.G527. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M. Leukotriene B4 potentiates colonic ulceration in the rat. Dig Dis Sci. 1990 May;35(5):622–629. doi: 10.1007/BF01540411. [DOI] [PubMed] [Google Scholar]
- Zifroni A., Treves A. J., Sachar D. B., Rachmilewitz D. Prostanoid synthesis by cultured intestinal epithelial and mononuclear cells in inflammatory bowel disease. Gut. 1983 Jul;24(7):659–664. doi: 10.1136/gut.24.7.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van 't Hof W., van Meer G. Generation of lipid polarity in intestinal epithelial (Caco-2) cells: sphingolipid synthesis in the Golgi complex and sorting before vesicular traffic to the plasma membrane. J Cell Biol. 1990 Sep;111(3):977–986. doi: 10.1083/jcb.111.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]


