Abstract
It has been shown that partial amino acid sequence homology between alpha gliadin and an early region protein (E1B-58 kDa) of adenovirus 12 results in immunological cross reaction. This led to the proposal that prior infection by adenovirus 12 could be associated with the development of coeliac disease. To examine this hypothesis, evidence was sought of persistent adenovirus 12 infection in the small intestinal mucosa of patients with coeliac disease. DNA isolated from biopsy samples from 24 control and 18 coeliac disease patients was analysed by the polymerase chain reaction for adenovirus 12 DNA encoding the E1B-58 kDa protein. Four of 18 coeliac disease and two of 24 control patients were positive. There is thus a low prevalence of this infection on both groups of patients but certainly no significantly increased incidence in coeliac disease. These results suggest that persistent adenovirus 12 infection is not a major element in the pathogenesis of coeliac disease.
Full text
PDF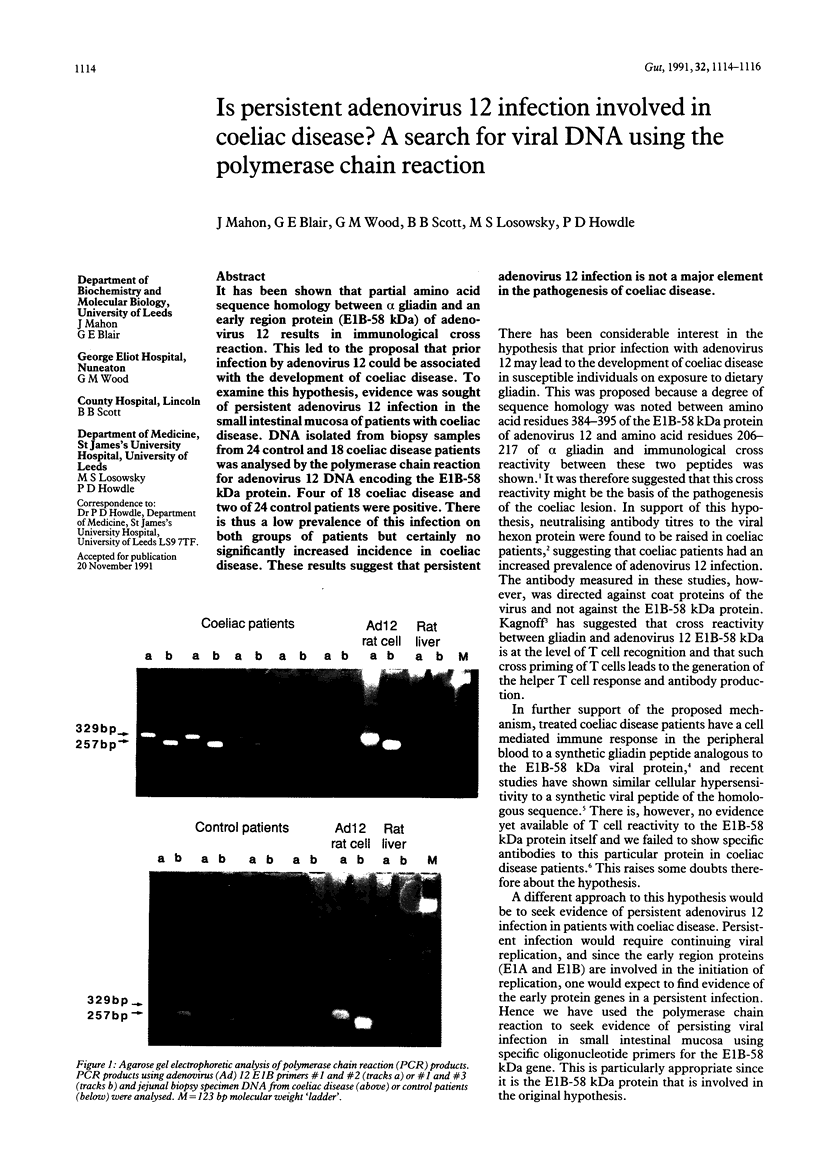


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bos J. L., Polder L. J., Bernards R., Schrier P. I., van den Elsen P. J., van der Eb A. J., van Ormondt H. The 2.2 kb E1b mRNA of human Ad12 and Ad5 codes for two tumor antigens starting at different AUG triplets. Cell. 1981 Nov;27(1 Pt 2):121–131. doi: 10.1016/0092-8674(81)90366-4. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Willcocks M. M., Mitchison H. C., Record C. O., Madeley C. R. Is a persistent adenovirus infection involved in coeliac disease? Gut. 1989 Nov;30(11):1563–1567. doi: 10.1136/gut.30.11.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdle P. D., Blair Zajdel M. E., Smart C. J., Trejdosiewicz L. K., Blair G. E., Losowky M. S. Lack of a serologic response to an E1B protein of adenovirus 12 in coeliac disease. Scand J Gastroenterol. 1989 Apr;24(3):282–286. doi: 10.3109/00365528909093047. [DOI] [PubMed] [Google Scholar]
- Jessberger R., Weisshaar B., Stabel S., Doerfler W. Arrangement and expression of integrated adenovirus type 12 DNA in the transformed hamster cell line HA12/7: amplification of Ad12 and c-myc DNAs and evidence for hybrid viral-cellular transcripts. Virus Res. 1989 Jun;13(2):113–128. doi: 10.1016/0168-1702(89)90010-5. [DOI] [PubMed] [Google Scholar]
- Kagnoff M. F., Austin R. K., Hubert J. J., Bernardin J. E., Kasarda D. D. Possible role for a human adenovirus in the pathogenesis of celiac disease. J Exp Med. 1984 Nov 1;160(5):1544–1557. doi: 10.1084/jem.160.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M. F. Celiac disease: adenovirus and alpha gliadin. Curr Top Microbiol Immunol. 1989;145:67–78. doi: 10.1007/978-3-642-74594-2_6. [DOI] [PubMed] [Google Scholar]
- Kagnoff M. F., Paterson Y. J., Kumar P. J., Kasarda D. D., Carbone F. R., Unsworth D. J., Austin R. K. Evidence for the role of a human intestinal adenovirus in the pathogenesis of coeliac disease. Gut. 1987 Aug;28(8):995–1001. doi: 10.1136/gut.28.8.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis J. A., Priddle J. D., Jewell D. P. Cell-mediated immunity to a synthetic gliadin peptide resembling a sequence from adenovirus 12. Lancet. 1987 Apr 18;1(8538):884–886. doi: 10.1016/s0140-6736(87)92859-5. [DOI] [PubMed] [Google Scholar]
- Kruijer W., van Schaik F. M., Speijer J. G., Sussenbach J. S. Structure and function of adenovirus DNA binding protein: comparison of the amino acid sequences of the Ad5 and Ad12 proteins derived from the nucleotide sequence of the corresponding genes. Virology. 1983 Jul 15;128(1):140–153. doi: 10.1016/0042-6822(83)90325-2. [DOI] [PubMed] [Google Scholar]
- Mantzaris G. J., Karagiannis J. A., Priddle J. D., Jewell D. P. Cellular hypersensitivity to a synthetic dodecapeptide derived from human adenovirus 12 which resembles a sequence of A-gliadin in patients with coeliac disease. Gut. 1990 Jun;31(6):668–673. doi: 10.1136/gut.31.6.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Peutherer J. F., Ludlam C. A., Bishop J. O., Brown A. J. Human immunodeficiency virus-infected individuals contain provirus in small numbers of peripheral mononuclear cells and at low copy numbers. J Virol. 1990 Feb;64(2):864–872. doi: 10.1128/jvi.64.2.864-872.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]