Abstract
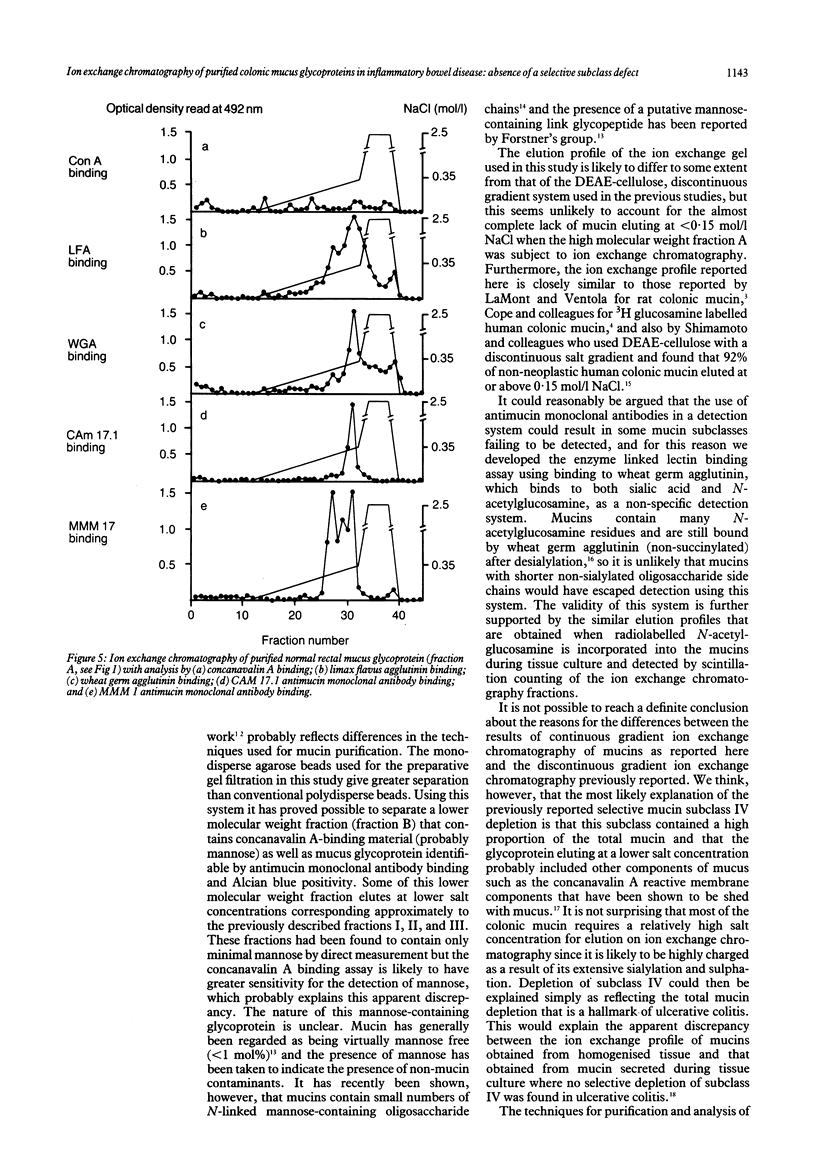
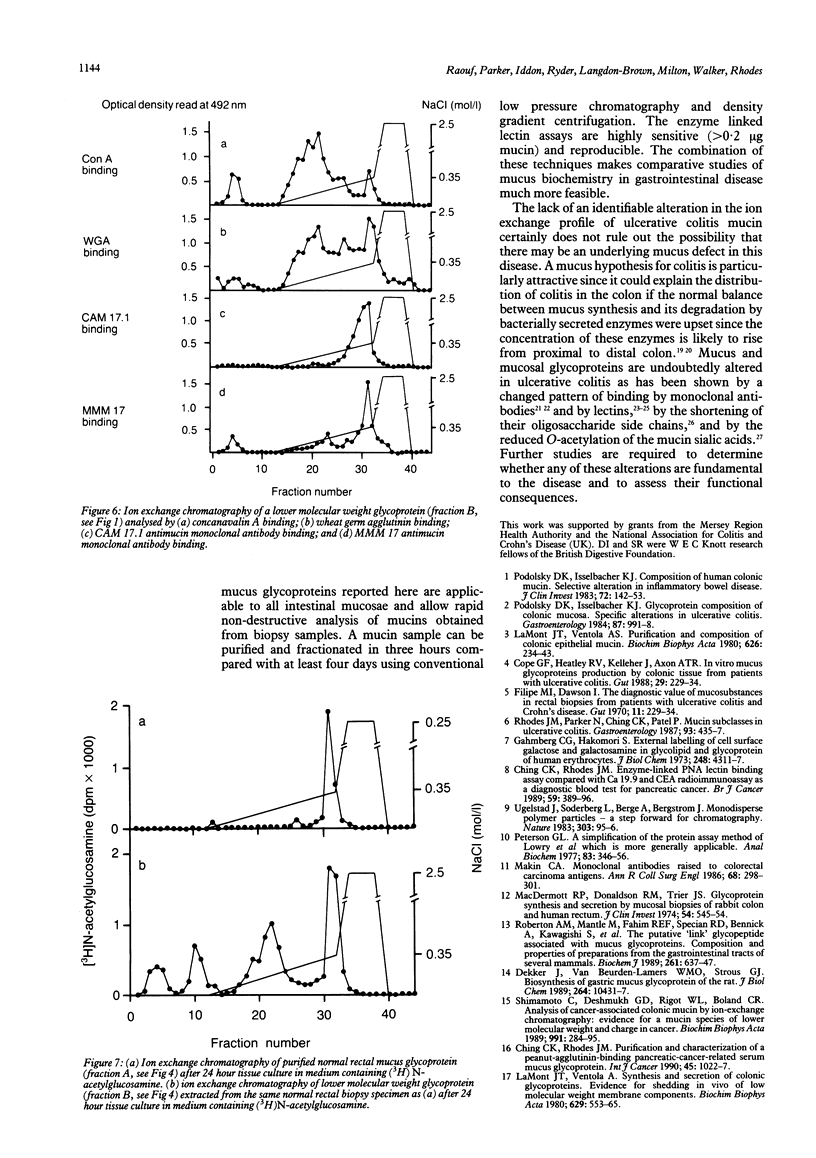
Previous reports of a selective mucin subclass defect in ulcerative colitis have been reassessed using high performance chromatography (Superose 6 and Mono Q) for mucin purification and fractionation coupled with analysis of the fractions obtained using a combination of enzyme linked lectin and mucin antibody assays. Mucin samples purified from snap frozen rectal biopsy specimens obtained from patients with ulcerative colitis (n = 12), Crohn's disease (n = 5), and non-inflammatory bowel disease control subjects (n = 9) were subject to ion exchange chromatography using a continuous 0-0.35 mol/l NaCl salt gradient with a final 2.5 mol/l NaCl step. In all samples the major proportion (mean (SD) 86.7 (8.9)%) of the mucin detectable by wheat germ agglutinin binding eluted between 0.15 and 0.35 mol/l NaCl with no significant difference in elution profile between ulcerative colitis and control subjects. Significant elution of glycoprotein at less than 0.15 mol/l NaCl did occur, however, when a lower molecular weight mucin containing fraction which contained concanavalin A positive (glucose or mannose containing) material was analysed similarly. Similar ion exchange profiles were obtained when (3H)N-acetylglucosamine labelled mucins were studied after tissue culture of rectal biopsy specimens. No significant alteration in the ion exchange profile of purified mucins in ulcerative colitis has been shown in these studies. It is possible that the previously reported relative depletion of mucin subclass IV (eluting with 0.20 mol/l NaCl) may simply have reflected mucin depletion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boland C. R., Lance P., Levin B., Riddell R. H., Kim Y. S. Abnormal goblet cell glycoconjugates in rectal biopsies associated with an increased risk of neoplasia in patients with ulcerative colitis: early results of a prospective study. Gut. 1984 Dec;25(12):1364–1371. doi: 10.1136/gut.25.12.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching C. K., Rhodes J. M. Purification and characterization of a peanut-agglutinin-binding pancreatic-cancer-related serum mucus glycoprotein. Int J Cancer. 1990 Jun 15;45(6):1022–1027. doi: 10.1002/ijc.2910450607. [DOI] [PubMed] [Google Scholar]
- Cope G. F., Heatley R. V., Kelleher J., Axon A. T. In vitro mucus glycoprotein production by colonic tissue from patients with ulcerative colitis. Gut. 1988 Feb;29(2):229–234. doi: 10.1136/gut.29.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culling C. F., Reid P. E., Dunn W. L. A histochemical comparison of the O-acylated sialic acids of the epithelial mucins in ulcerative colitis, Crohn's disease, and normal controls. J Clin Pathol. 1979 Dec;32(12):1272–1277. doi: 10.1136/jcp.32.12.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J., Van Beurden-Lamers W. M., Strous G. J. Biosynthesis of gastric mucus glycoprotein of the rat. J Biol Chem. 1989 Jun 25;264(18):10431–10437. [PubMed] [Google Scholar]
- Filipe M. I., Dawson I. The diagnostic value of mucosubstances in rectal biopsies from patients with ulcerative colitis and Crohn's disease. Gut. 1970 Mar;11(3):229–234. doi: 10.1136/gut.11.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Jacobs L. R., Huber P. W. Regional distribution and alterations of lectin binding to colorectal mucin in mucosal biopsies from controls and subjects with inflammatory bowel diseases. J Clin Invest. 1985 Jan;75(1):112–118. doi: 10.1172/JCI111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMont J. T., Ventola A. S. Purification and composition of colonic epithelial mucin. Biochim Biophys Acta. 1980 Nov 20;626(1):234–243. doi: 10.1016/0005-2795(80)90214-7. [DOI] [PubMed] [Google Scholar]
- Lamont J. T., Ventola A. Synthesis and secretion of colonic glycoproteins: evidence for shedding in vivo of low molecular weight membrane components. Biochim Biophys Acta. 1980 May 22;629(3):553–565. [PubMed] [Google Scholar]
- MacDermott R. P., Donaldson R. M., Jr, Trier J. S. Glycoprotein synthesis and secretion by mucosal biopsies of rabbit colon and human rectum. J Clin Invest. 1974 Sep;54(3):545–554. doi: 10.1172/JCI107791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin C. A. Monoclonal antibodies raised to colorectal carcinoma antigens. Ann R Coll Surg Engl. 1986 Nov;68(6):298–301. [PMC free article] [PubMed] [Google Scholar]
- Miller R. S., Hoskins L. C. Mucin degradation in human colon ecosystems. Fecal population densities of mucin-degrading bacteria estimated by a "most probable number" method. Gastroenterology. 1981 Oct;81(4):759–765. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A. Alterations in mucosal content of colonic glycoconjugates in inflammatory bowel disease defined by monoclonal antibodies. Gastroenterology. 1988 Aug;95(2):379–387. doi: 10.1016/0016-5085(88)90494-5. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A. Emergence of antigenic glycoprotein structures in ulcerative colitis detected through monoclonal antibodies. Gastroenterology. 1988 Aug;95(2):371–378. doi: 10.1016/0016-5085(88)90493-3. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Composition of human colonic mucin. Selective alteration in inflammatory bowel disease. J Clin Invest. 1983 Jul;72(1):142–153. doi: 10.1172/JCI110952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Glycoprotein composition of colonic mucosa. Specific alterations in ulcerative colitis. Gastroenterology. 1984 Nov;87(5):991–998. [PubMed] [Google Scholar]
- Rhodes J. M., Black R. R., Gallimore R., Savage A. Histochemical demonstration of desialation and desulphation of normal and inflammatory bowel disease rectal mucus by faecal extracts. Gut. 1985 Dec;26(12):1312–1318. doi: 10.1136/gut.26.12.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. M., Black R. R., Savage A. Altered lectin binding by colonic epithelial glycoconjugates in ulcerative colitis and Crohn's disease. Dig Dis Sci. 1988 Nov;33(11):1359–1363. doi: 10.1007/BF01536988. [DOI] [PubMed] [Google Scholar]
- Rhodes J. M., Parker N., Ching C. K., Patel P. Mucin subclasses in ulcerative colitis. Gastroenterology. 1987 Aug;93(2):435–437. doi: 10.1016/0016-5085(87)91054-7. [DOI] [PubMed] [Google Scholar]
- Roberton A. M., Mantle M., Fahim R. E., Specian R. D., Bennick A., Kawagishi S., Sherman P., Forstner J. F. The putative 'link' glycopeptide associated with mucus glycoproteins. Composition and properties of preparations from the gastrointestinal tracts of several mammals. Biochem J. 1989 Jul 15;261(2):637–647. doi: 10.1042/bj2610637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto C., Deshmukh G. D., Rigot W. L., Boland C. R. Analysis of cancer-associated colonic mucin by ion-exchange chromatography: evidence for a mucin species of lower molecular charge and weight in cancer. Biochim Biophys Acta. 1989 May 31;991(2):284–295. doi: 10.1016/0304-4165(89)90118-9. [DOI] [PubMed] [Google Scholar]
- Smith A. C., Podolsky D. K. Biosynthesis and secretion of human colonic mucin glycoproteins. J Clin Invest. 1987 Aug;80(2):300–307. doi: 10.1172/JCI113073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teague R. H., Fraser D., Clamp J. R. Changes in monosaccharide content of mucous glycoproteins in ulcerative colitis. Br Med J. 1973 Jun 16;2(5867):645–646. doi: 10.1136/bmj.2.5867.645. [DOI] [PMC free article] [PubMed] [Google Scholar]