Abstract
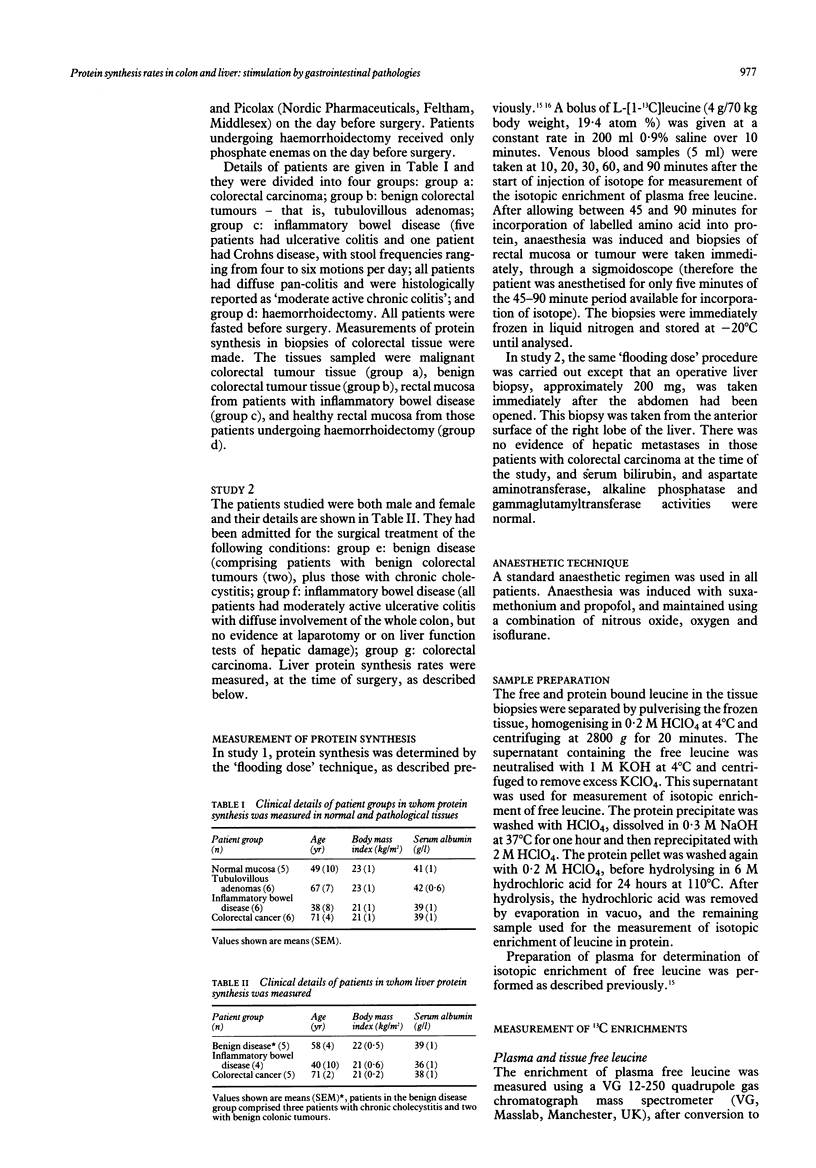
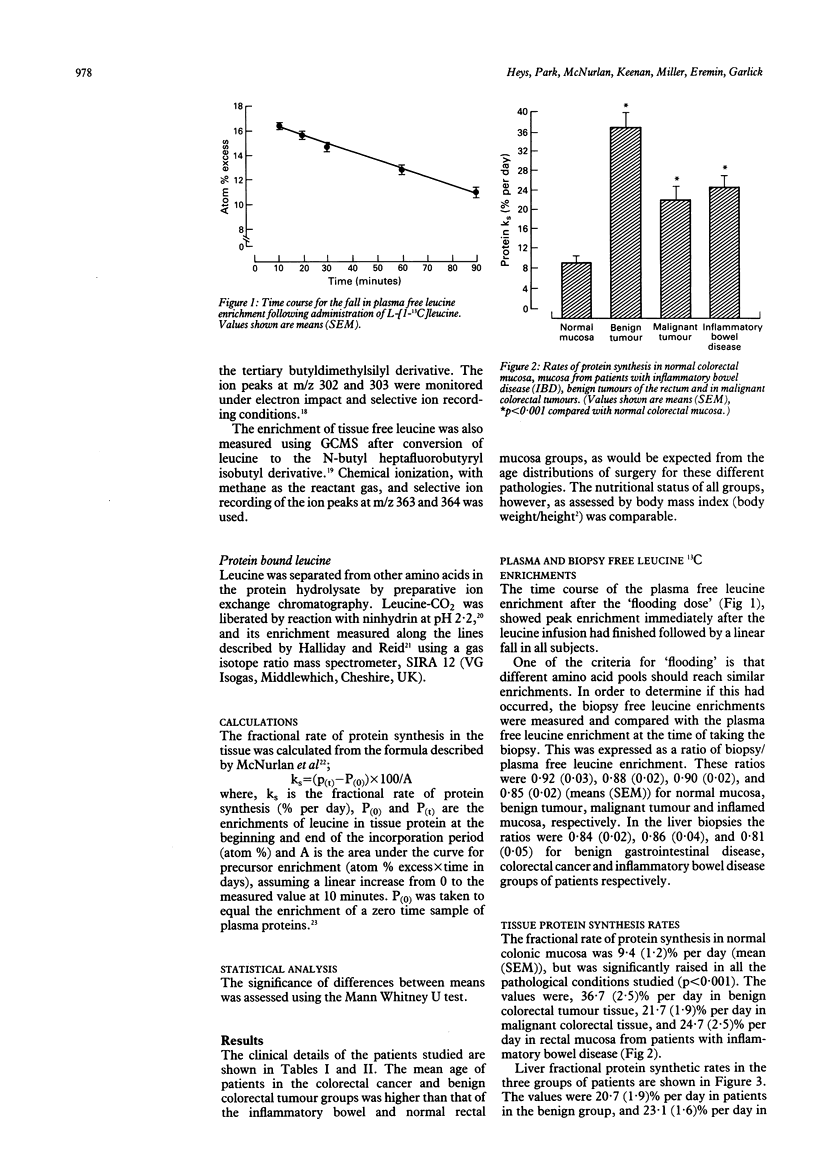
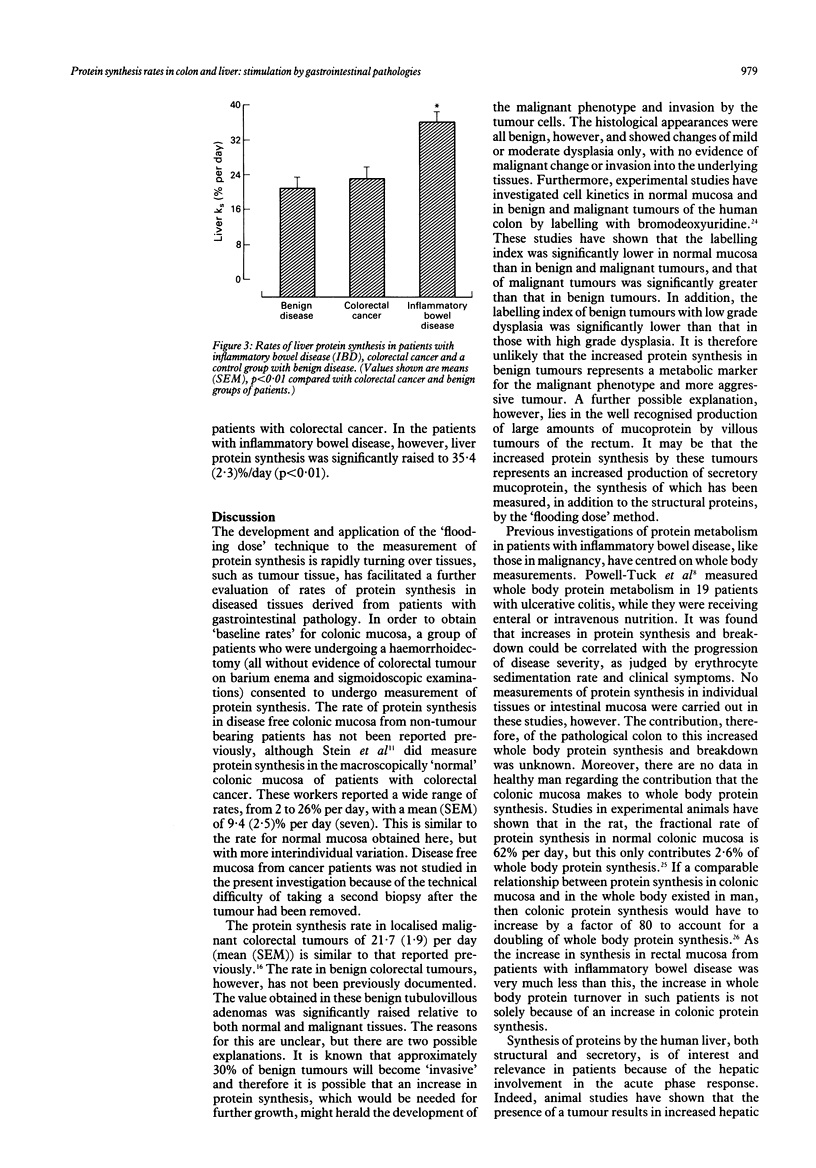
Rates of protein synthesis in vivo in normal and pathological tissues of the gastrointestinal tract, were measured using the 'flooding dose' technique with the stable isotope L-[1-13C] leucine. The rate of protein synthesis in normal colonic mucosa was 9.4 (1.2)% (mean (SEM)) per day but was significantly raised in benign and malignant colorectal tumour tissue, and in colonic mucosa from patients with inflammatory bowel disease (p less than 0.001). Furthermore, the rate of protein synthesis was significantly greater in benign colorectal tumour tissue, 36.7 (2.5)% per day, than that in either malignant tumour tissue, 21.7 (1.9)% per day, or in inflammatory bowel disease mucosa, 24.7 (2.5)% per day (means (SEM) p less than 0.001). Liver protein synthesis rates were also measured in separate groups of patients with benign disease of the gastrointestinal tract, in patients with colorectal carcinoma, and in patients with inflammatory bowel disease. The fractional rate of liver protein synthesis was 20.7 (1.9)% per day in patients with benign disease and 23.1 (1.6)% per day in patients with colorectal cancer. In patients with inflammatory bowel disease, however, liver protein synthesis was significantly increased to 35.4 (2.3)% per day (means (SEM) p less than 0.01).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballmer P. E., McNurlan M. A., Milne E., Heys S. D., Buchan V., Calder A. G., Garlick P. J. Measurement of albumin synthesis in humans: a new approach employing stable isotopes. Am J Physiol. 1990 Dec;259(6 Pt 1):E797–E803. doi: 10.1152/ajpendo.1990.259.6.E797. [DOI] [PubMed] [Google Scholar]
- Bennet W. M., Connacher A. A., Scrimgeour C. M., Smith K., Rennie M. J. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: studies of incorporation of [1-13C]leucine. Clin Sci (Lond) 1989 Apr;76(4):447–454. doi: 10.1042/cs0760447. [DOI] [PubMed] [Google Scholar]
- Brennan M. F. Uncomplicated starvation versus cancer cachexia. Cancer Res. 1977 Jul;37(7 Pt 2):2359–2364. [PubMed] [Google Scholar]
- Buckell N. A., Lennard-Jones J. E., Hernandez M. A., Kohn J., Riches P. G., Wadsworth J. Measurement of serum proteins during attacks of ulcerative colitis as a guide to patient management. Gut. 1979 Jan;20(1):22–27. doi: 10.1136/gut.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOKE W. T., FOWLER D. I., COX E. V., GADDIE R., MEYNELL M. J. The clinical significance of seromucoids in regional ileitis and ulcerative colitis. Gastroenterology. 1958 May;34(5):910–919. [PubMed] [Google Scholar]
- Calder A. G., Smith A. Stable isotope ratio analysis of leucine and ketoisocaproic acid in blood plasma by gas chromatography/mass spectrometry. Use of tertiary butyldimethylsilyl derivatives. Rapid Commun Mass Spectrom. 1988 Jan;2(1):14–16. doi: 10.1002/rcm.1290020105. [DOI] [PubMed] [Google Scholar]
- Charters Y., Grimble R. F. Effect of recombinant human tumour necrosis factor alpha on protein synthesis in liver, skeletal muscle and skin of rats. Biochem J. 1989 Mar 1;258(2):493–497. doi: 10.1042/bj2580493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P. W., Edwards R. H., Rennie M. J., Souhami R. L., Halliday D. Protein synthesis in muscle measured in vivo in cachectic patients with cancer. Br Med J (Clin Res Ed) 1984 Sep 8;289(6445):584–586. doi: 10.1136/bmj.289.6445.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., Wernerman J., McNurlan M. A., Essen P., Lobley G. E., Milne E., Calder G. A., Vinnars E. Measurement of the rate of protein synthesis in muscle of postabsorptive young men by injection of a 'flooding dose' of [1-13C]leucine. Clin Sci (Lond) 1989 Sep;77(3):329–336. doi: 10.1042/cs0770329. [DOI] [PubMed] [Google Scholar]
- Glass R. E., Fern E. B., Garlick P. J. Whole-body protein turnover before and after resection of colorectal tumours. Clin Sci (Lond) 1983 Jan;64(1):101–108. doi: 10.1042/cs0640101. [DOI] [PubMed] [Google Scholar]
- Halliday D., Read W. W. Mass spectrometric assay of stable isotopic enrichment for the estimation of protein turnover in man. Proc Nutr Soc. 1981 Sep;40(3):321–324. doi: 10.1079/pns19810048. [DOI] [PubMed] [Google Scholar]
- Heber D., Chlebowski R. T., Ishibashi D. E., Herrold J. N., Block J. B. Abnormalities in glucose and protein metabolism in noncachectic lung cancer patients. Cancer Res. 1982 Nov;42(11):4815–4819. [PubMed] [Google Scholar]
- Herrmann V. M., Garnick M. B., Moore F. D., Wilmore D. W. Effect of cytotoxic agents on protein kinetics in patients with metastatic cancer. Surgery. 1981 Aug;90(2):381–387. [PubMed] [Google Scholar]
- Heys S. D., McNurlan M. A., Park K. G., Milne E., Garlick P. J. Baseline measurements for stable isotope studies: an alternative to biopsy. Biomed Environ Mass Spectrom. 1990 Mar;19(3):176–178. doi: 10.1002/bms.1200190314. [DOI] [PubMed] [Google Scholar]
- Heys S. D., Norton A. C., Dundas C. R., Eremin O., Ferguson K., Garlick P. J. Anaesthetic agents and their effect on tissue protein synthesis in the rat. Clin Sci (Lond) 1989 Dec;77(6):651–655. doi: 10.1042/cs0770651. [DOI] [PubMed] [Google Scholar]
- Heys S. D., Park K. G., McNurlan M. A., Calder A. G., Buchan V., Blessing K., Eremin O., Garlick P. J. Measurement of tumour protein synthesis in vivo in human colorectal and breast cancer and its variability in separate biopsies from the same tumour. Clin Sci (Lond) 1991 Jun;80(6):587–593. doi: 10.1042/cs0800587. [DOI] [PubMed] [Google Scholar]
- Jepson M. M., Pell J. M., Bates P. C., Millward D. J. The effects of endotoxaemia on protein metabolism in skeletal muscle and liver of fed and fasted rats. Biochem J. 1986 Apr 15;235(2):329–336. doi: 10.1042/bj2350329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubega J., Davies T. J. A comparison of serum mucoprotein with serum alpha 1 acid glycoprotein, haptoglobin, and alpha 1 antitrypsin assays in monitoring inflammatory bowel disease. Clin Chim Acta. 1990 Apr 13;188(1):59–69. doi: 10.1016/0009-8981(90)90146-j. [DOI] [PubMed] [Google Scholar]
- MacKenzie S. L., Tenaschuk D. Gas-liquid chromatography of N-heptafluorobutyryl isobutyl esters of amino acids. J Chromatogr. 1974 Oct 9;97(1):19–24. doi: 10.1016/s0021-9673(01)97579-x. [DOI] [PubMed] [Google Scholar]
- McNurlan M. A., Essen P., Heys S. D., Buchan V., Garlick P. J., Wernerman J. Measurement of protein synthesis in human skeletal muscle: further investigation of the flooding technique. Clin Sci (Lond) 1991 Oct;81(4):557–564. doi: 10.1042/cs0810557. [DOI] [PubMed] [Google Scholar]
- McNurlan M. A., Pain V. M., Garlick P. J. Conditions that alter rates of tissue protein synthesis in vivo. Biochem Soc Trans. 1980 Jun;8(3):283–285. doi: 10.1042/bst0080283. [DOI] [PubMed] [Google Scholar]
- McNurlan M. A., Tomkins A. M., Garlick P. J. The effect of starvation on the rate of protein synthesis in rat liver and small intestine. Biochem J. 1979 Feb 15;178(2):373–379. doi: 10.1042/bj1780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville S., McNurlan M. A., Calder A. G., Garlick P. J. Increased protein turnover despite normal energy metabolism and responses to feeding in patients with lung cancer. Cancer Res. 1990 Feb 15;50(4):1125–1131. [PubMed] [Google Scholar]
- Mullen J. L., Buzby G. P., Gertner M. H., Stein T. P., Hargrove W. C., Oram-Smith J., Rosato E. F. Protein synthesis dynamics in human gastrointestinal malignancies. Surgery. 1980 Mar;87(3):331–338. [PubMed] [Google Scholar]
- Pain V. M., Randall D. P., Garlick P. J. Protein synthesis in liver and skeletal muscle of mice bearing an ascites tumor. Cancer Res. 1984 Mar;44(3):1054–1057. [PubMed] [Google Scholar]
- Powell-Tuck J., Garlick P. J., Lennard-Jones J. E., Waterlow J. C. Rates of whole body protein synthesis and breakdown increase with the severity of inflammatory bowel disease. Gut. 1984 May;25(5):460–464. doi: 10.1136/gut.25.5.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell-Tuck J. Protein metabolism in inflammatory bowel disease. Gut. 1986 Nov;27 (Suppl 1):67–71. doi: 10.1136/gut.27.suppl_1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risio M., Coverlizza S., Ferrari A., Candelaresi G. L., Rossini F. P. Immunohistochemical study of epithelial cell proliferation in hyperplastic polyps, adenomas, and adenocarcinomas of the large bowel. Gastroenterology. 1988 Apr;94(4):899–906. doi: 10.1016/0016-5085(88)90545-8. [DOI] [PubMed] [Google Scholar]
- Stein T. P., Mullen J. L., Oram-Smith J. C., Rosato E. F., Wallace H. W., Hargrove W. C., 3rd Relative rates of tumor, normal gut, liver, and fibrinogen protein synthesis in man. Am J Physiol. 1978 Jun;234(6):E648–E652. doi: 10.1152/ajpendo.1978.234.6.E648. [DOI] [PubMed] [Google Scholar]
- Weeke B., Jarnum S. Serum concentration of 19 serum proteins in Crohn's disease and ulcerative colitis. Gut. 1971 Apr;12(4):297–302. doi: 10.1136/gut.12.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch C. S., Adams M., Wakefield E. G. METABOLIC STUDIES ON CHRONIC ULCERATIVE COLITIS. J Clin Invest. 1937 Jan;16(1):161–168. doi: 10.1172/JCI100835. [DOI] [PMC free article] [PubMed] [Google Scholar]


