Abstract
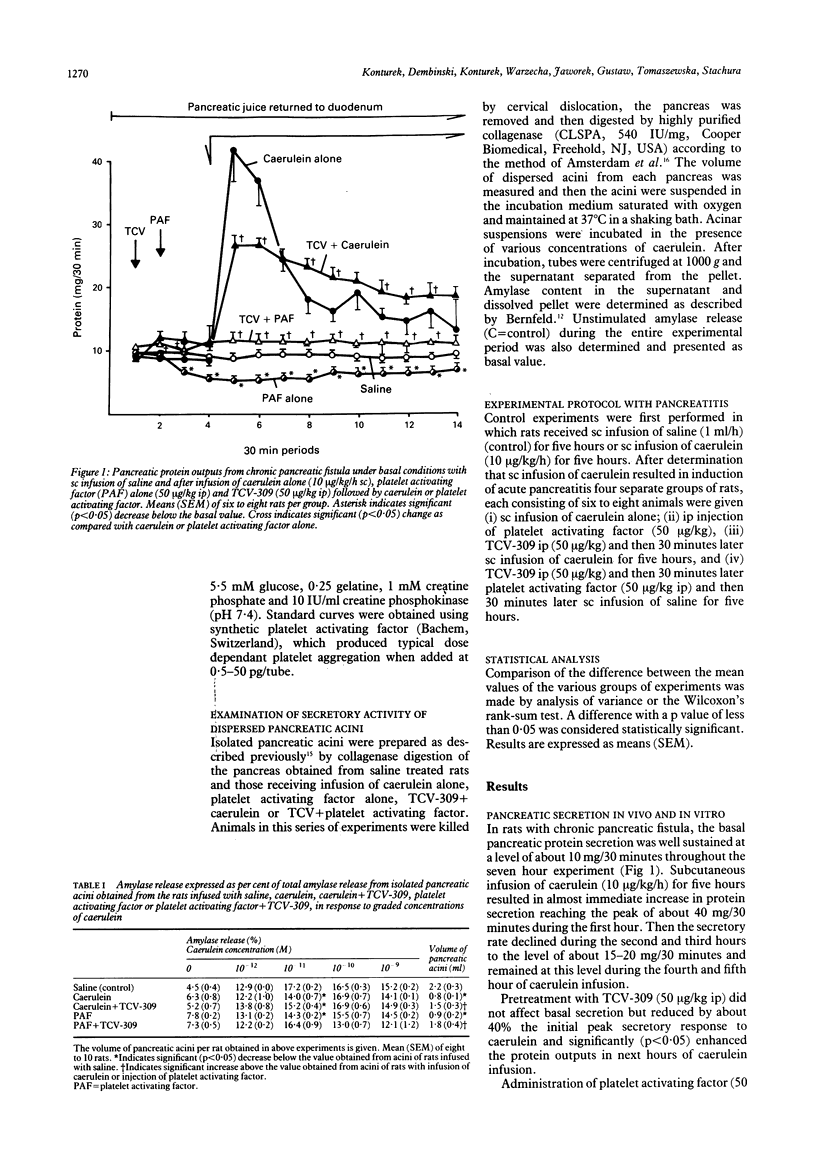
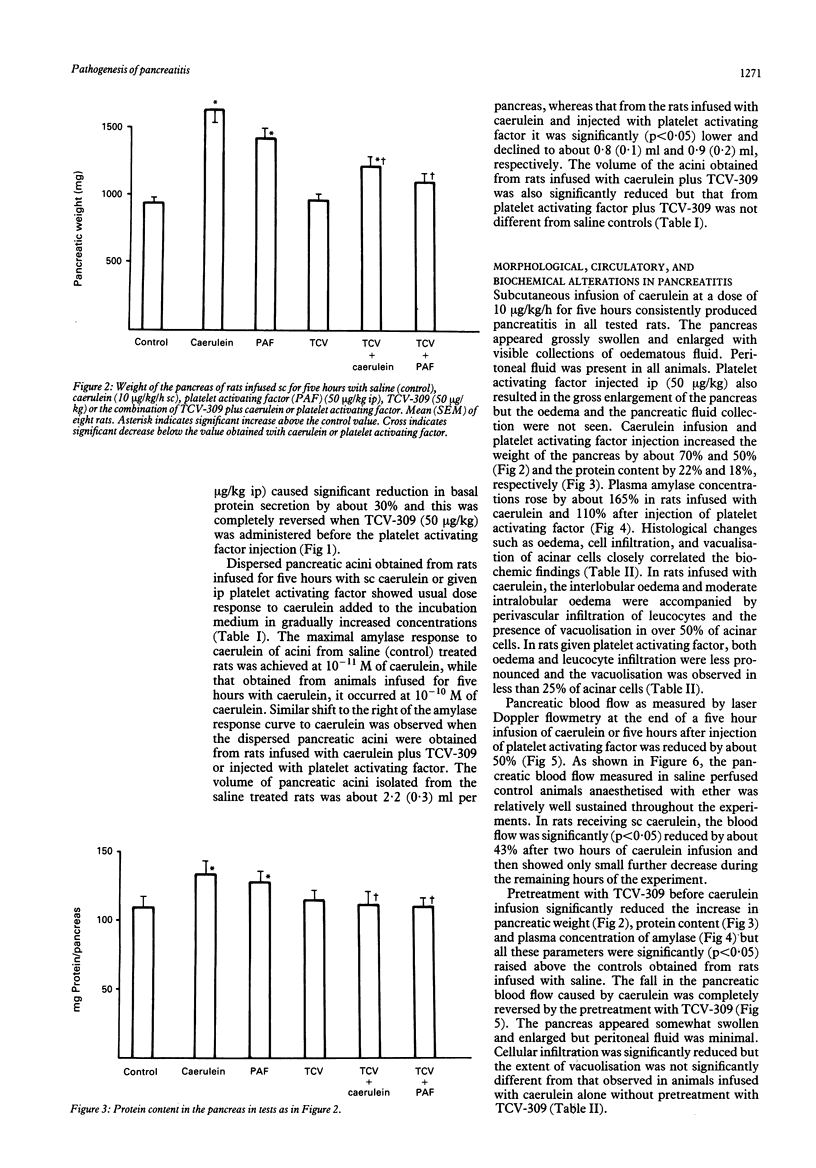
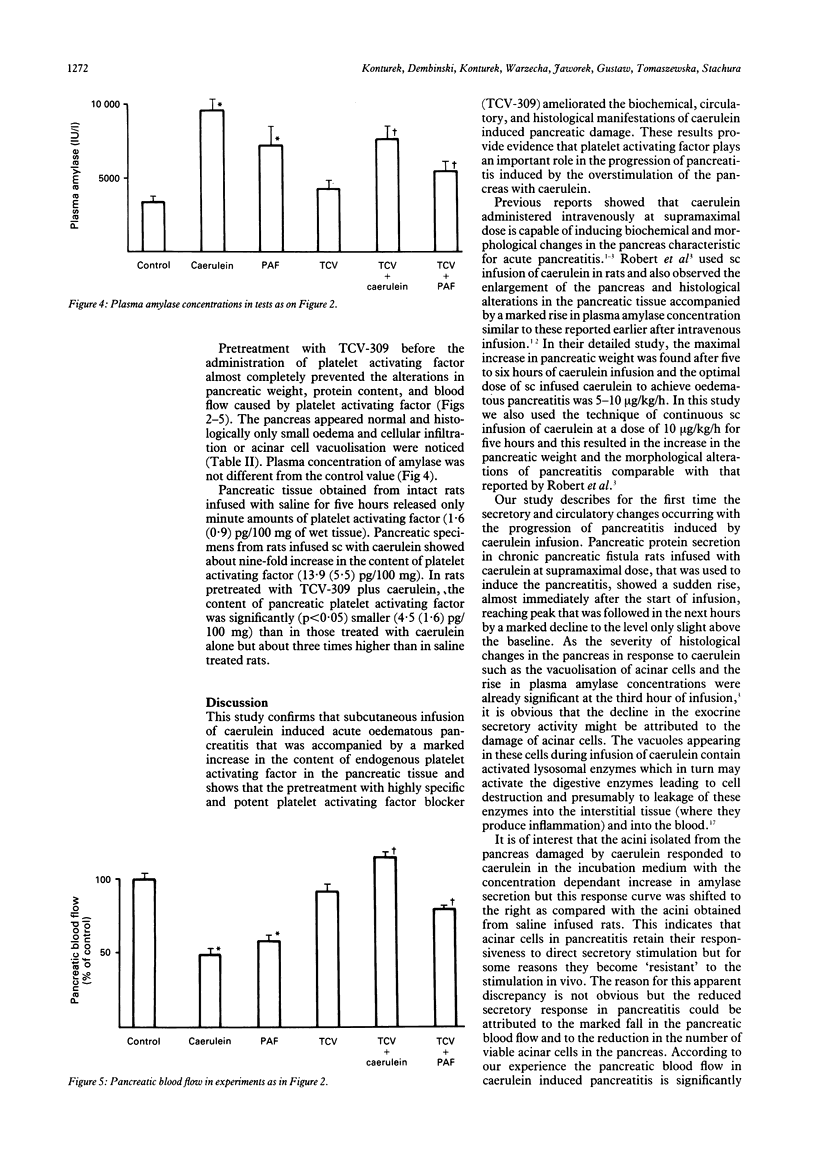
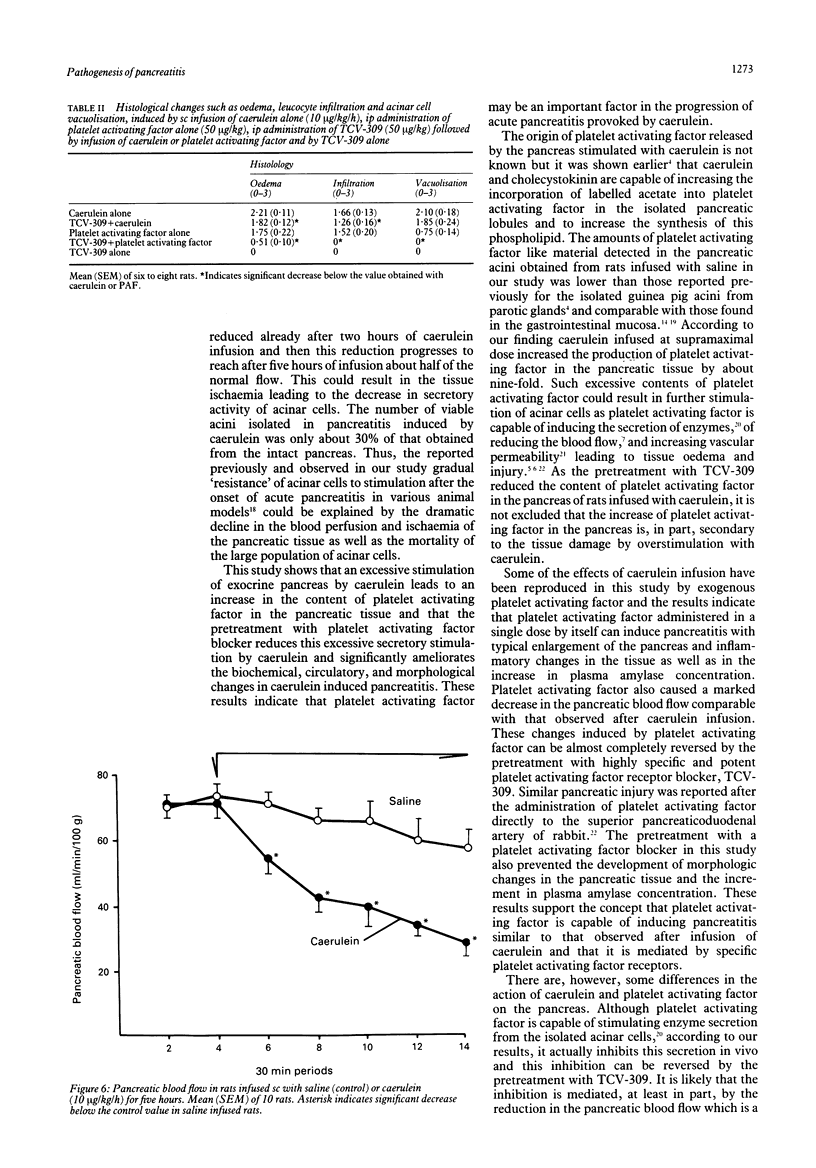
The importance of platelet activating factor in acute pancreatitis was examined by determining the tissue content of endogenous platelet activating factor and the protective effects of TCV-309, a highly selective platelet activating factor blocker, against caerulein induced pancreatitis in rats. Infusion of caerulein (10 micrograms/kg/h) for five hours resulted in about 70% increase in pancreatic weight, 22% rise in protein content, 50% reduction in tissue blood flow, nine fold increase in tissue level of platelet activating factor and 165% rise in plasma amylase as well as histological evidence of acute pancreatitis. Such infusion of caerulein in chronic pancreatic fistula rats caused a marked increase in protein output from basal secretion of 10 mg/30 minutes to 40 mg/30 minutes in the first hour of infusion followed by a decline in protein output to 15-20 mg/30 minutes in the following hours of the experiment. Exogenous platelet activating factor (50 micrograms/kg) injected ip produced similar alterations in weight, protein content, blood flow, and histology of the pancreas but the increment in serum amylase was significantly smaller and pancreatic secretion was reduced below the basal level. TCV-309 (50 micrograms/kg) given ip before caerulein or platelet activating factor administration significantly reduced the biochemical and morphological alterations caused by caerulein and abolished those induced by exogenous platelet activating factor. These results indicate that platelet activating factor plays an important role in the pathogenesis of acute pancreatitis probably by reducing the blood flow and increasing vascular permeability in the pancreas.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnet J., Loiseau A. M., Orvoen M., Bessin P. Platelet-activating factor acether (PAF-acether) involvement in acute inflammatory and pain processes. Agents Actions. 1981 Dec;11(6-7):559–562. doi: 10.1007/BF01978740. [DOI] [PubMed] [Google Scholar]
- Casals-Stenzel J., Heuer H. Pharmacology of PAF antagonists. Prog Biochem Pharmacol. 1988;22:58–65. [PubMed] [Google Scholar]
- Dembinski A., Jaworek J., Konturek P. K., Konturek S. J., Warzecha Z. Cholecystokinin receptor antagonism by peptidergic and non-peptidergic agents in rat pancreas. J Physiol. 1989 Apr;411:419–435. doi: 10.1113/jphysiol.1989.sp017581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliakim R., Karmeli F., Razin E., Rachmilewitz D. Role of platelet-activating factor in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine and prednisolone. Gastroenterology. 1988 Nov;95(5):1167–1172. doi: 10.1016/0016-5085(88)90346-0. [DOI] [PubMed] [Google Scholar]
- Emanuelli G., Montrucchio G., Gaia E., Dughera L., Corvetti G., Gubetta L. Experimental acute pancreatitis induced by platelet activating factor in rabbits. Am J Pathol. 1989 Feb;134(2):315–326. [PMC free article] [PubMed] [Google Scholar]
- Hsueh W., Gonzalez-Crussi F., Arroyave J. L. Sequential release of leukotrienes and norepinephrine in rat bowel after platelet-activating factor. A mechanistic study of platelet-activating factor-induced bowel necrosis. Gastroenterology. 1988 Jun;94(6):1412–1418. doi: 10.1016/0016-5085(88)90680-4. [DOI] [PubMed] [Google Scholar]
- Humphrey D. M., McManus L. M., Hanahan D. J., Pinckard R. N. Morphologic basis of increased vascular permeability induced by acetyl glyceryl ether phosphorylcholine. Lab Invest. 1984 Jan;50(1):16–25. [PubMed] [Google Scholar]
- Konturek S. J., Krzyzek E., Bilski J. The importance of gastric secretion in the feedback control of interdigestive and postprandial pancreatic secretion in rats. Regul Pept. 1991 Oct 1;36(1):85–97. doi: 10.1016/0167-0115(91)90197-o. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Pawlik W., Czarnobilski K., Gustaw P., Jaworek J., Beck G., Jendralla H. Effects of leukotriene C4 on pancreatic secretion and circulation in dogs. Am J Physiol. 1988 Jun;254(6 Pt 1):G849–G855. doi: 10.1152/ajpgi.1988.254.6.G849. [DOI] [PubMed] [Google Scholar]
- Kubes P., Ibbotson G., Russell J., Wallace J. L., Granger D. N. Role of platelet-activating factor in ischemia/reperfusion-induced leukocyte adherence. Am J Physiol. 1990 Aug;259(2 Pt 1):G300–G305. doi: 10.1152/ajpgi.1990.259.2.G300. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampel M., Kern H. F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977 Mar 11;373(2):97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- Niederau C., Niederau M., Lüthen R., Strohmeyer G., Ferrell L. D., Grendell J. H. Pancreatic exocrine secretion in acute experimental pancreatitis. Gastroenterology. 1990 Oct;99(4):1120–1127. doi: 10.1016/0016-5085(90)90633-c. [DOI] [PubMed] [Google Scholar]
- Robert A., Lum J. T., Lancaster C., Olafsson A. S., Kolbasa K. P., Nezamis J. E. Prevention by prostaglandins of caerulein-induced pancreatitis in rats. Lab Invest. 1989 May;60(5):677–691. [PubMed] [Google Scholar]
- Söling H. D., Eibl H., Fest W. Acetylcholine-like effects of 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine ('platelet-activating factor') and its analogues in exocrine secretory glands. Eur J Biochem. 1984 Oct 1;144(1):65–72. doi: 10.1111/j.1432-1033.1984.tb08431.x. [DOI] [PubMed] [Google Scholar]
- Söling H. D., Fest W. Synthesis of 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine (platelet-activating factor) in exocrine glands and its control by secretagogues. J Biol Chem. 1986 Oct 25;261(30):13916–13922. [PubMed] [Google Scholar]
- Takatani M., Maezaki N., Imura Y., Terashita Z., Nishikawa K., Tsushima S. Platelet activating factor (PAF) antagonists: development of a highly potent PAF antagonist, TCV-309. Adv Prostaglandin Thromboxane Leukot Res. 1991;21B:943–946. [PubMed] [Google Scholar]
- Wallace J. L., Steel G., Whittle B. J., Lagente V., Vargaftig B. Evidence for platelet-activating factor as a mediator of endotoxin-induced gastrointestinal damage in the rat. Effects of three platelet-activating factor antagonists. Gastroenterology. 1987 Oct;93(4):765–773. doi: 10.1016/0016-5085(87)90438-0. [DOI] [PubMed] [Google Scholar]
- Watanabe O., Baccino F. M., Steer M. L., Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984 Apr;246(4 Pt 1):G457–G467. doi: 10.1152/ajpgi.1984.246.4.G457. [DOI] [PubMed] [Google Scholar]


