Abstract
1 The aim of the studies was to develop a new methodology to estimate the pharmacodynamic properties and potency of angiotensin converting enzyme (ACE) inhibitors in man.
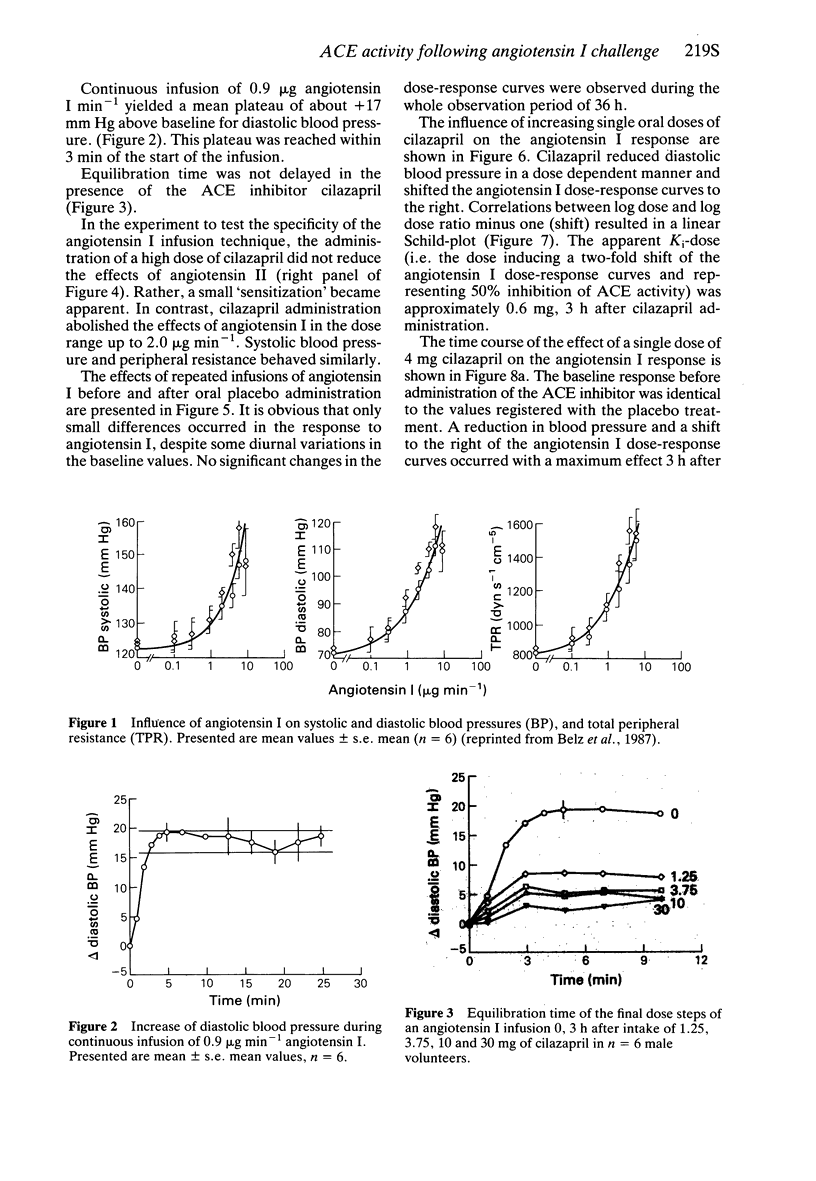
2 Angiotensin I dose-response curves were derived by continuous infusion of angiotensin I in increasing dose steps; steady state was reached within 3 min.
3 Interaction between angiotensin I (agonist) and ACE inhibitors (antagonist) was characterized according to Schild-plot methodology by measuring agonist dose-response curves using diastolic blood pressure in the absence and the presence of varying doses of the antagonist.
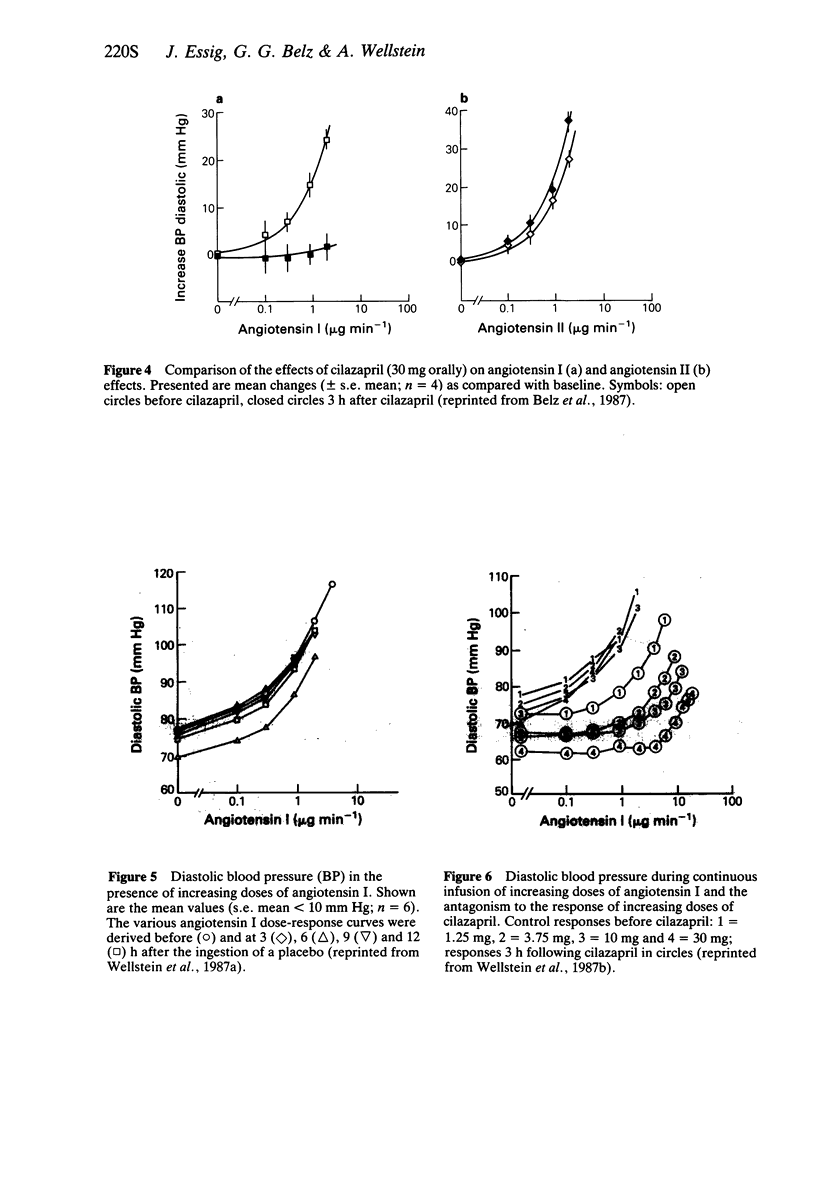
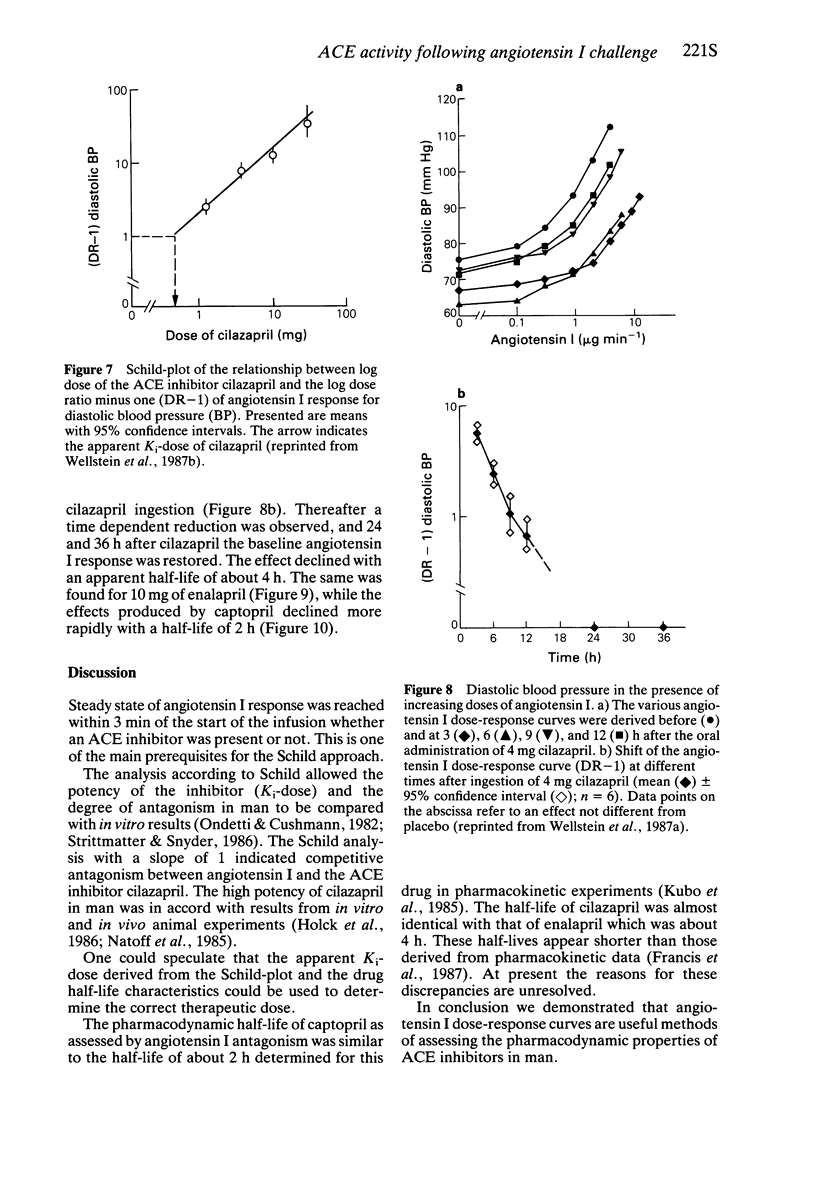
4 Cilazapril shifted the angiotensin I dose-response curves to the right. A twofold shift (apparent Ki-dose) was observed with approximately 0.6 mg cilazapril.
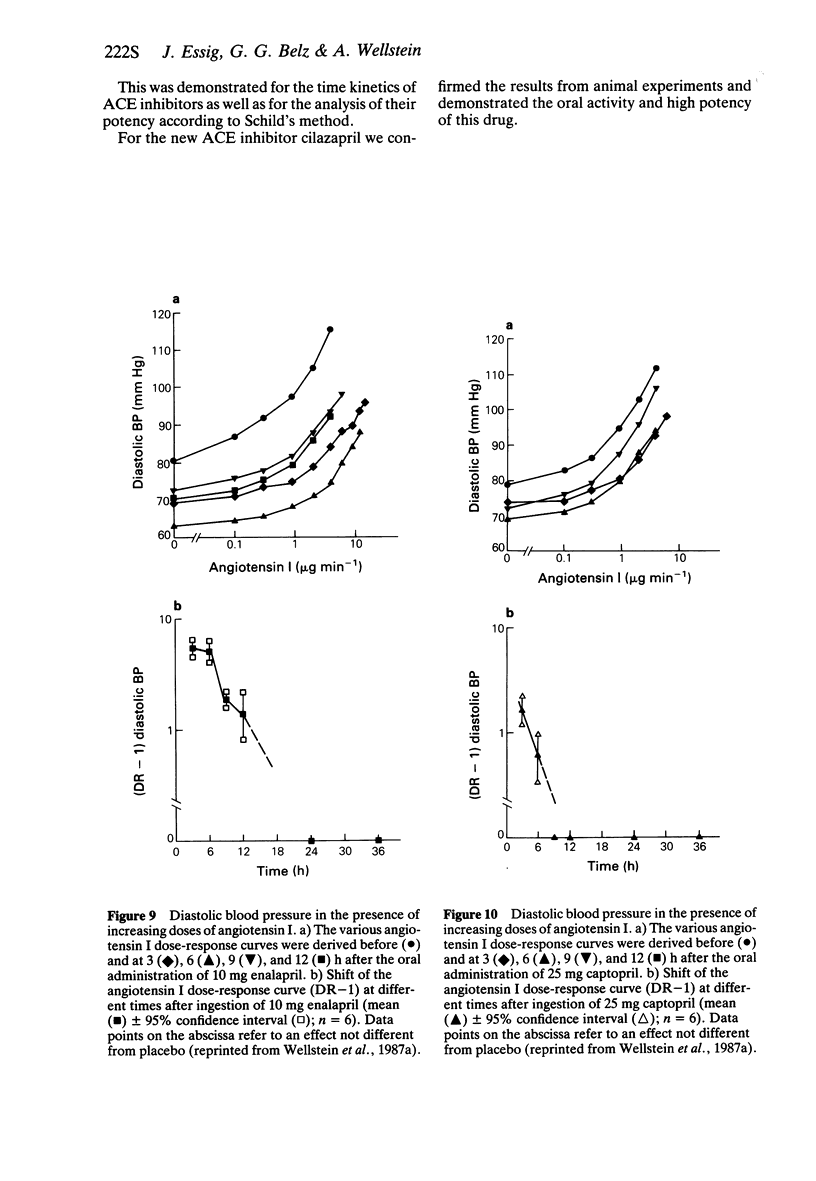
5 The effect of angiotensin I on diastolic blood pressure was determined before and up to 36 h after administration of 25 mg captopril, 10 mg enalapril, 4 mg cilazapril and a placebo orally. The pharmacodynamic half-life of captopril was about 2 h, whereas the effect of enalapril and cilazapril was about 4 h.
6 Angiotensin I dose-response curves are useful methods of investigating the pharmacodynamic properties of ACE-inhibitors in man.
Keywords: kinetics, pharmacodynamic properties, angiotensin I, ACE inhibitors
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz G. G., Essig J., Wellstein A. Hemodynamic responses to angiotensin I in normal volunteers and the antagonism by the angiotensin-converting enzyme inhibitor cilazapril. J Cardiovasc Pharmacol. 1987 Feb;9(2):219–224. doi: 10.1097/00005344-198702000-00015. [DOI] [PubMed] [Google Scholar]
- Biollaz J., Burnier M., Turini G. A., Brunner D. B., Porchet M., Gomez H. J., Jones K. H., Ferber F., Abrams W. B., Gavras H. Three new long-acting converting-enzyme inhibitors: relationship between plasma converting-enzyme activity and response to angiotensin I. Clin Pharmacol Ther. 1981 May;29(5):665–670. doi: 10.1038/clpt.1981.92. [DOI] [PubMed] [Google Scholar]
- Bussien J. P., Nussberger J., Porchet M., Waeber B., Brunner H. R., Perisic M., Tansey M. J., Bomm M., Hajdu P. The effect of the converting enzyme inhibitor HOE 498 on the renin angiotensin system of normal volunteers. Naunyn Schmiedebergs Arch Pharmacol. 1985 Mar;329(1):63–69. doi: 10.1007/BF00695194. [DOI] [PubMed] [Google Scholar]
- Francis R. J., Brown A. N., Kler L., Fasanella d'Amore T., Nussberger J., Waeber B., Brunner H. R. Pharmacokinetics of the converting enzyme inhibitor cilazapril in normal volunteers and the relationship to enzyme inhibition: development of a mathematical model. J Cardiovasc Pharmacol. 1987 Jan;9(1):32–38. [PubMed] [Google Scholar]
- Given B. D., Taylor T., Hollenberg N. K., Williams G. H. Duration of action and short-term hormonal responses to enalapril (MK 421) in normal subjects. J Cardiovasc Pharmacol. 1984 May-Jun;6(3):436–441. doi: 10.1097/00005344-198405000-00010. [DOI] [PubMed] [Google Scholar]
- Holck M., Fischli W., Hefti F., Gerold M. Cardiovascular effects of the new angiotensin-converting-enzyme inhibitor, cilazapril, in anesthetized and conscious dogs. J Cardiovasc Pharmacol. 1986 Jan-Feb;8(1):99–108. doi: 10.1097/00005344-198601000-00016. [DOI] [PubMed] [Google Scholar]
- Kubo S. H., Cody R. J. Clinical pharmacokinetics of the angiotensin converting enzyme inhibitors. A review. Clin Pharmacokinet. 1985 Sep-Oct;10(5):377–391. doi: 10.2165/00003088-198510050-00001. [DOI] [PubMed] [Google Scholar]
- Natoff I. L., Nixon J. S., Francis R. J., Klevans L. R., Brewster M., Budd J., Patel A. T., Wenger J., Worth E. Biological properties of the angiotensin-converting enzyme inhibitor cilazapril. J Cardiovasc Pharmacol. 1985 May-Jun;7(3):569–580. doi: 10.1097/00005344-198505000-00025. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Cushman D. W. Enzymes of the renin-angiotensin system and their inhibitors. Annu Rev Biochem. 1982;51:283–308. doi: 10.1146/annurev.bi.51.070182.001435. [DOI] [PubMed] [Google Scholar]
- Strittmatter S. M., Snyder S. H. Characterization of angiotensin converting enzyme by [3H]captopril binding. Mol Pharmacol. 1986 Feb;29(2):142–148. [PubMed] [Google Scholar]
- Wellstein A., Belz G. G., Palm D. Beta adrenoceptor subtype binding activity in plasma and beta blockade by propranolol and beta-1 selective bisoprolol in humans. Evaluation with Schild-plots. J Pharmacol Exp Ther. 1988 Jul;246(1):328–337. [PubMed] [Google Scholar]
- Wellstein A., Essig J., Belz G. G. A method for estimating the potency of angiotensin-converting enzyme inhibitors in man. Br J Clin Pharmacol. 1987 Sep;24(3):397–399. doi: 10.1111/j.1365-2125.1987.tb03188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellstein A., Essig J., Belz G. G. Inhibition of angiotensin-I response by cilazapril and its time course in normal volunteers. Clin Pharmacol Ther. 1987 Jun;41(6):639–644. doi: 10.1038/clpt.1987.89. [DOI] [PubMed] [Google Scholar]
- Wellstein A., Palm D., Pitschner H. F., Belz G. G. Receptor binding of propranolol is the missing link between plasma concentration kinetics and the effect-time course in man. Eur J Clin Pharmacol. 1985;29(2):131–147. doi: 10.1007/BF00547412. [DOI] [PubMed] [Google Scholar]


