Abstract
1. The pharmacokinetics of the angiotensin converting enzyme inhibitor, lisinopril, were studied in an open, randomized, balanced, two-period, crossover design in 12 in-patients with stable, chronic congestive heart failure (CHF). 2. To evaluate the pharmacokinetics of lisinopril in CHF, lisinopril was administered orally (10 mg) and intravenously (5 mg) in each patient. Each dose was followed by a 72 h period with frequent blood sampling and fractional urine collections for radioimmunoassay of lisinopril. 3. Mean urinary recovery of lisinopril was 15 and 88% following oral and intravenous administration, respectively; absorption/bioavailability of lisinopril based on urinary recovery ratios was 16%, less than that found in normal subjects. 4. Serum concentrations of lisinopril following intravenous administration were higher in this study than those previously observed in normal subjects. 5. The results of this study suggest a reduced absorption of lisinopril in CHF and altered disposition, possibly associated with age as well as the disease state.
Full text
PDF

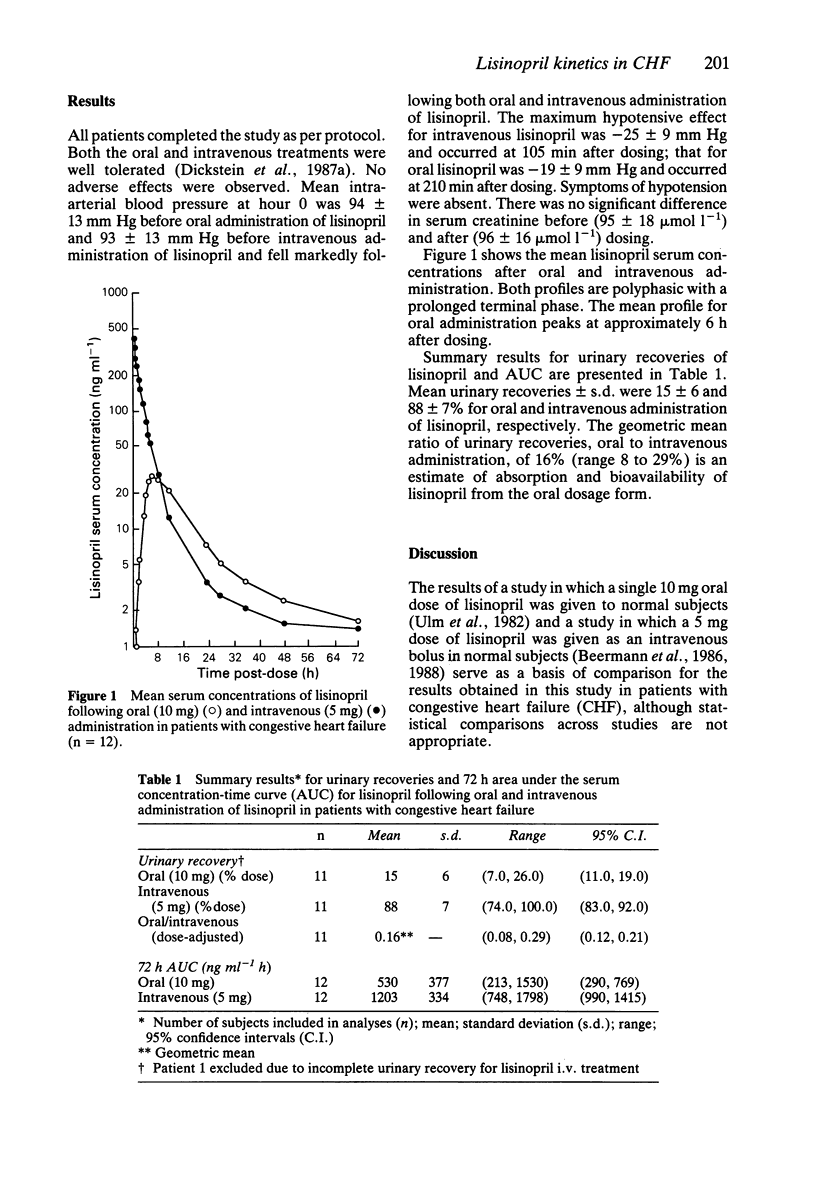


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
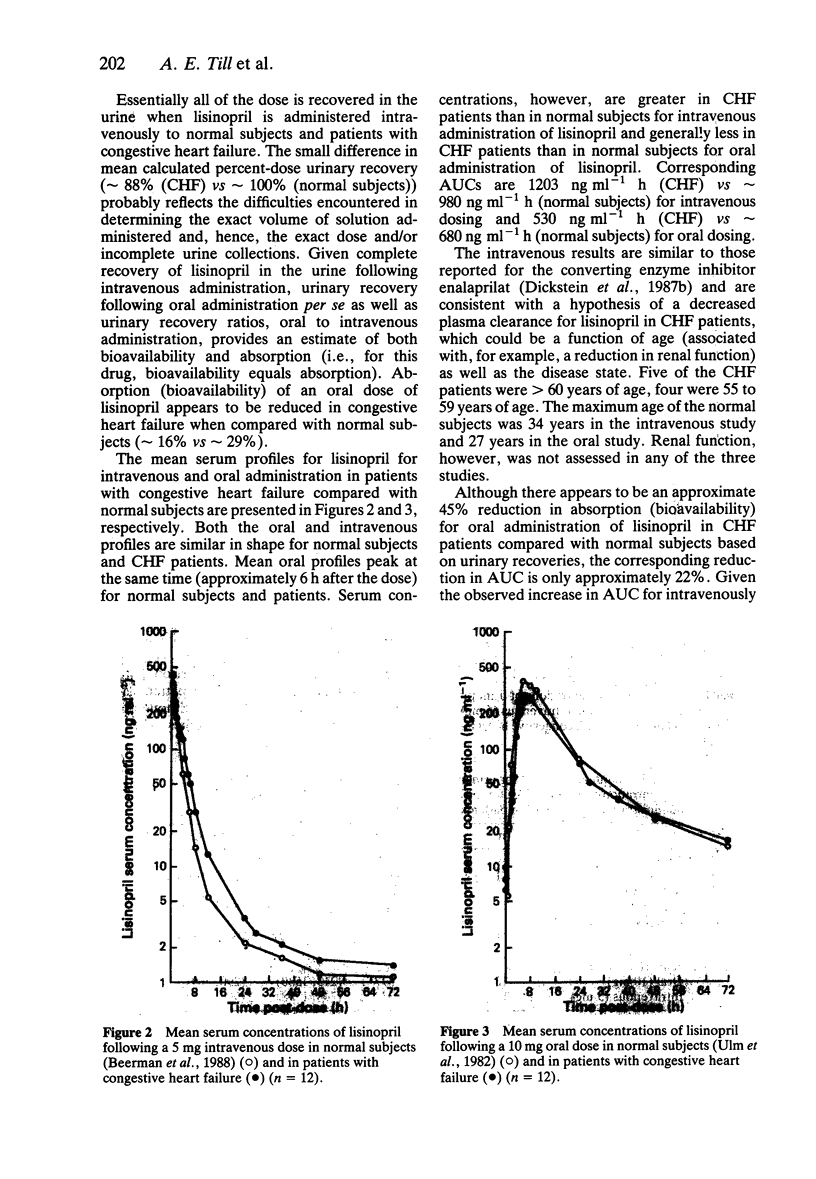
- Dickstein K., Aarsland T., Tjelta K., Cirillo V. J., Gomez H. J. A comparison of hypotensive responses after oral and intravenous administration of enalapril and lisinopril in chronic heart failure. J Cardiovasc Pharmacol. 1987 Jun;9(6):705–710. doi: 10.1097/00005344-198706000-00011. [DOI] [PubMed] [Google Scholar]
- Dickstein K., Aarsland T., Woie L., Abrahamsen A. M., Fyhrquist F., Cummings S., Gomex H. J., Hagen E., Kristianson K. Acute hemodynamic and hormonal effects of lisinopril (MK-521) in congestive heart failure. Am Heart J. 1986 Jul;112(1):121–129. doi: 10.1016/0002-8703(86)90689-7. [DOI] [PubMed] [Google Scholar]
- Dickstein K., Till A. E., Aarsland T., Tjelta K., Abrahamsen A. M., Kristianson K., Gomez H. J., Gregg H., Hichens M. The pharmacokinetics of enalapril in hospitalized patients with congestive heart failure. Br J Clin Pharmacol. 1987 Apr;23(4):403–410. doi: 10.1111/j.1365-2125.1987.tb03069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIZZLE J. E. THE TWO-PERIOD CHANGE-OVER DESIGN AN ITS USE IN CLINICAL TRIALS. Biometrics. 1965 Jun;21:467–480. [PubMed] [Google Scholar]
- Karlberg B. E., Fyhrquist F., Grönhagen-Riska C., Tikkanen I., Ohman K. P. Enalapril and lisinopril in renovascular hypertension--antihypertensive and hormonal effects of two new angiotensin-converting-enzyme (ACE) inhibitors. A preliminary report. Scand J Urol Nephrol Suppl. 1984;79:103–106. [PubMed] [Google Scholar]
- Till A. E., Gomez H. J., Hichens M., Bolognese J. A., McNabb W. R., Brooks B. A., Noormohamed F., Lant A. F. Pharmacokinetics of repeated single oral doses of enalapril maleate (MK-421) in normal volunteers. Biopharm Drug Dispos. 1984 Jul-Sep;5(3):273–280. doi: 10.1002/bdd.2510050309. [DOI] [PubMed] [Google Scholar]
- Ueda C. T., Dzindzio B. S. Bioavailability of quinidine in congestive heart failure. Br J Clin Pharmacol. 1981 Jun;11(6):571–577. doi: 10.1111/j.1365-2125.1981.tb01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm E. H., Hichens M., Gomez H. J., Till A. E., Hand E., Vassil T. C., Biollaz J., Brunner H. R., Schelling J. L. Enalapril maleate and a lysine analogue (MK-521): disposition in man. Br J Clin Pharmacol. 1982 Sep;14(3):357–362. doi: 10.1111/j.1365-2125.1982.tb01991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


