Abstract
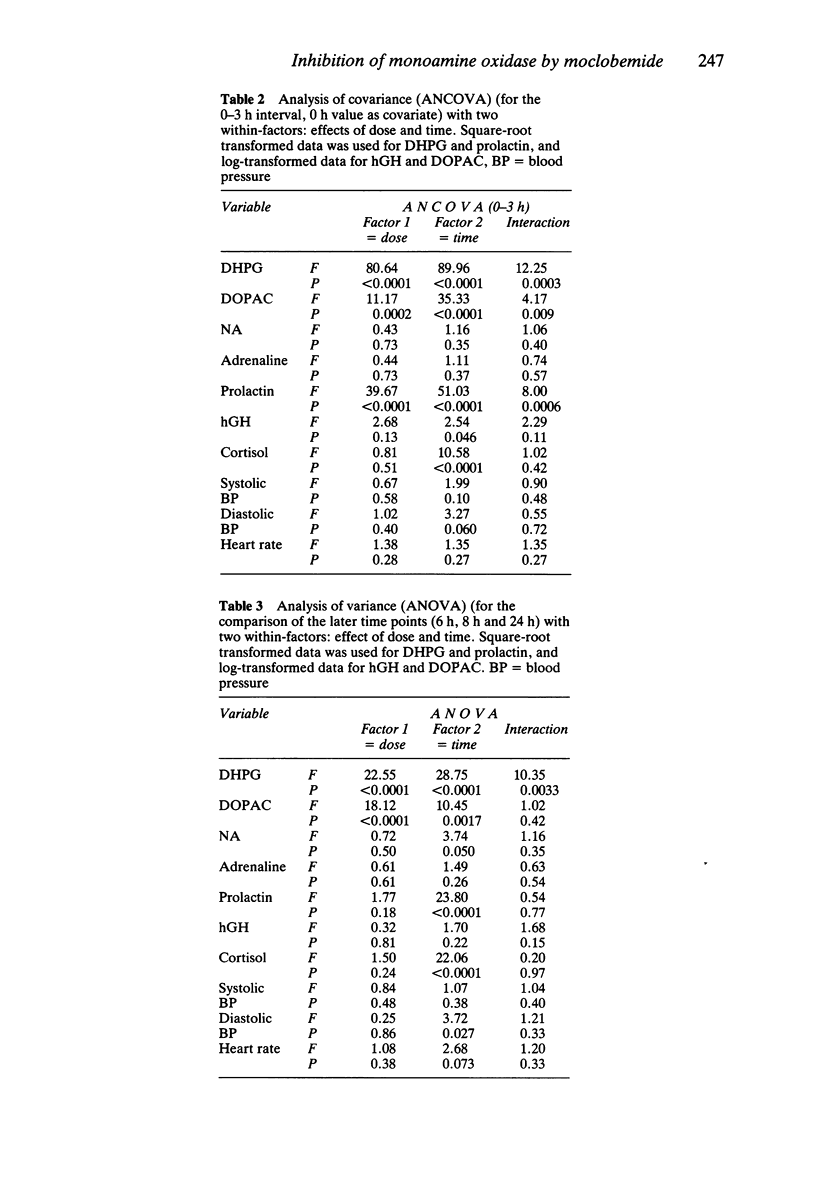
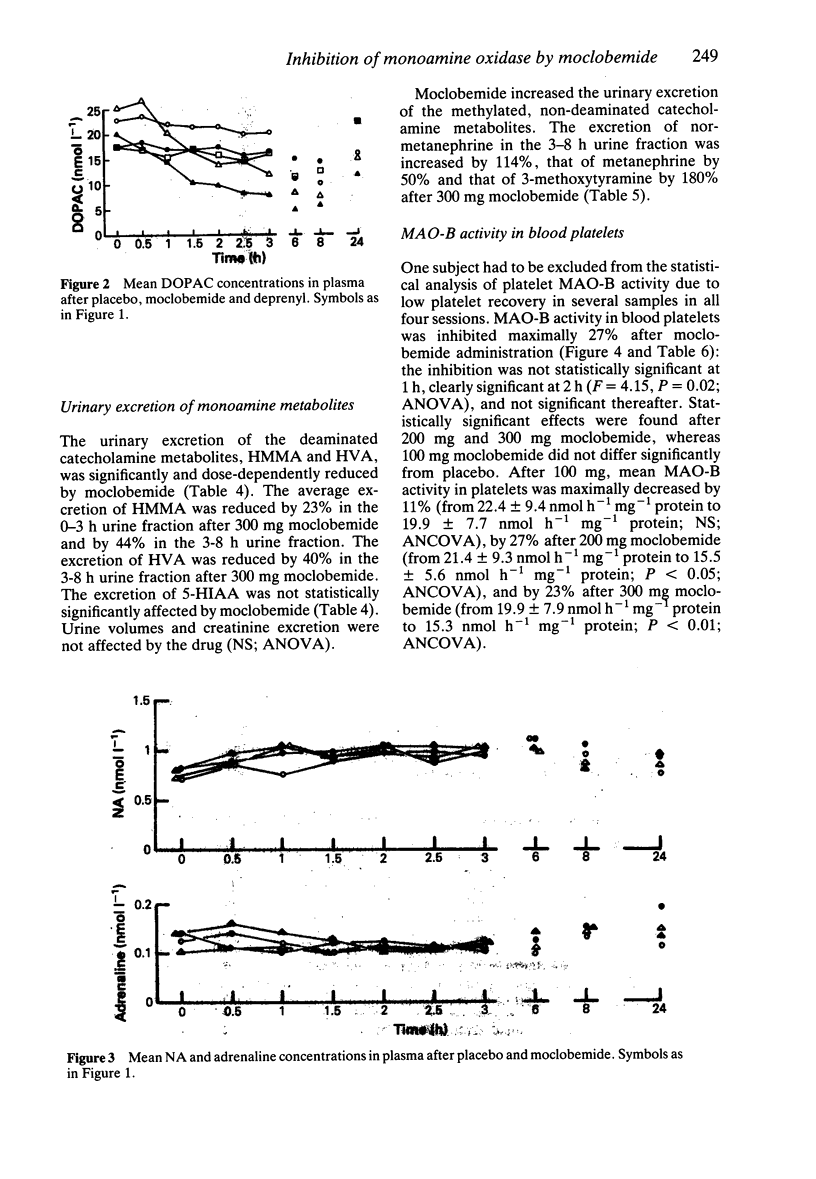
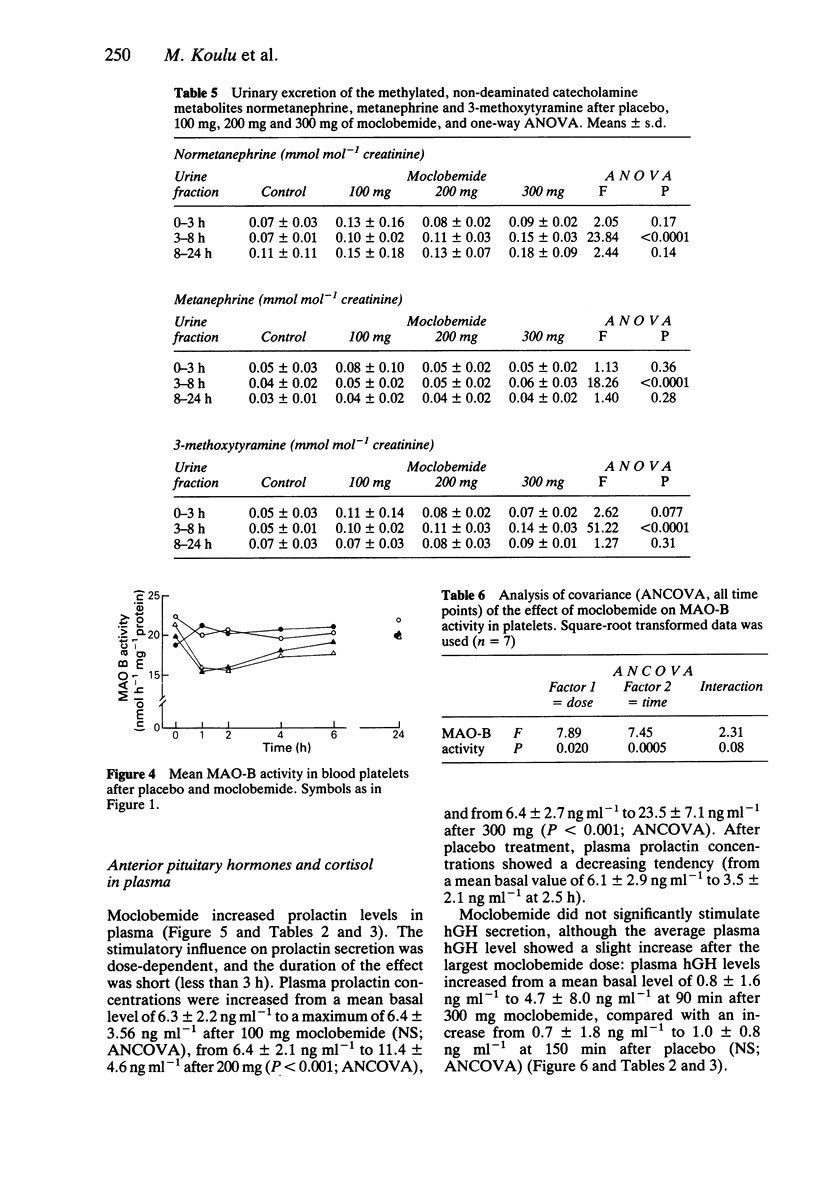
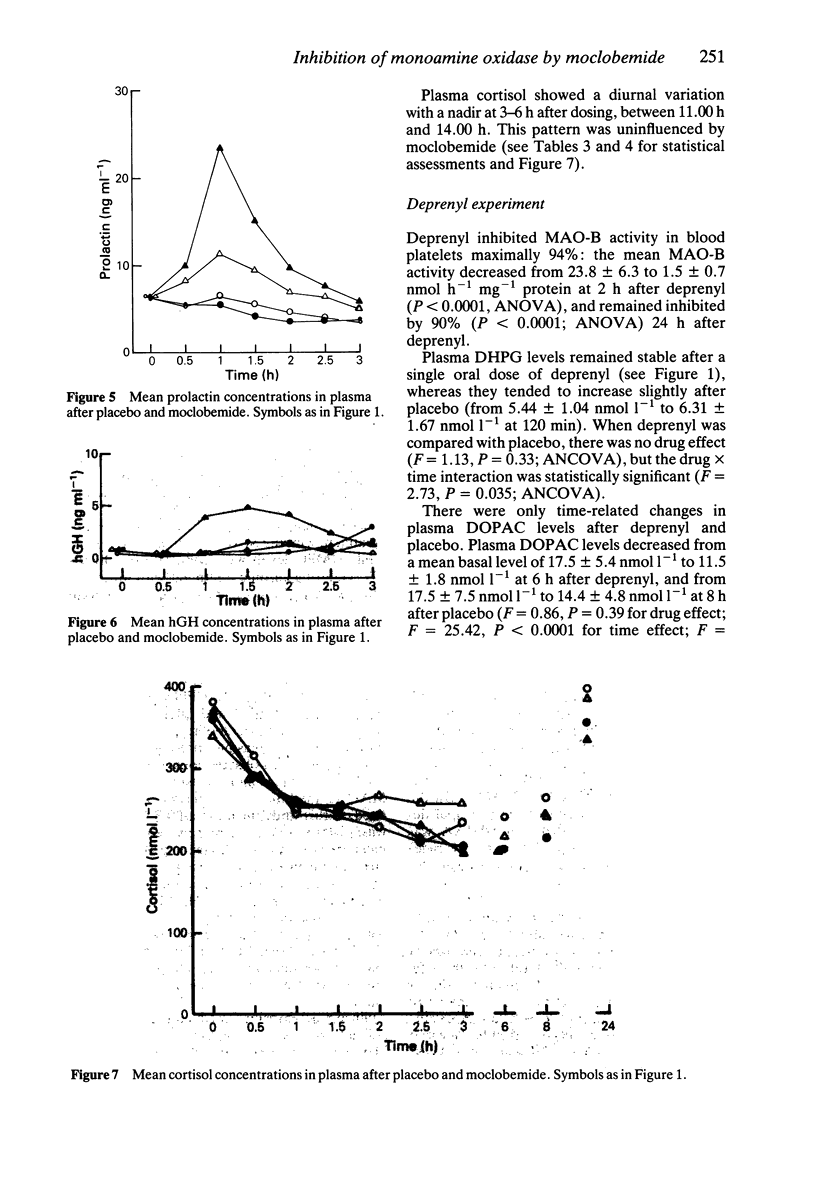
1. Single oral doses (100, 200 and 300 mg) of moclobemide, a reversible inhibitor of monoamine oxidase (MAO) with predominant effects on the A-type of the enzyme, were administered to eight young, healthy male volunteers in a double-blind, random-order, placebo-controlled study. The investigation was thereafter continued in an open fashion by administering a single 10 mg dose of the MAO-B inhibitor deprenyl to the same subjects. 2. Deamination of catecholamines was powerfully and dose-dependently inhibited by moclobemide, as evidenced by up to 40% decreases in the urinary excretion of deaminated catecholamine metabolites, corresponding increases in the excretion of non-deaminated, methylated metabolites, and up to 79% average decreases in the plasma concentration of 3,4-dihydroxyphenylglycol (DHPG), a deaminated metabolite of noradrenaline (NA), and up to 75% average decreases in the plasma concentrations of 3,4-dihydroxyphenylacetic acid (DOPAC), a deaminated metabolite of dopamine. The urinary excretion of 5-hydroxyindoleacetic acid (5-HIAA) was only slightly reduced. In contrast, deprenyl, in a dose which almost totally inhibited MAO-B activity in blood platelets, did not appreciably affect the plasma concentrations of DHPG or DOPAC. 3. Due to the rapid, reversible, dose-dependent and MAO-A specific effect of moclobemide on plasma concentrations of DHPG, it is suggested that DHPG in plasma may be a useful indicator of the magnitude and duration of MAO-A inhibition in man. 4. Sympatho-adrenal function at rest was not significantly altered by moclobemide, as judged by unchanged plasma catecholamine concentrations and stable blood pressure and heart rate recordings. 5. Monoamine oxidase type B activity in blood platelets was slightly (less than 30%) and transiently inhibited after moclobemide. 6. The secretion of prolactin was dose-dependently stimulated by moclobemide, whereas the plasma concentrations of growth hormone (hGH) and cortisol remained unchanged.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. M., Cowen P. J. Clomipramine enhances prolactin and growth hormone responses to L-tryptophan. Psychopharmacology (Berl) 1986;89(1):131–133. doi: 10.1007/BF00175205. [DOI] [PubMed] [Google Scholar]
- Bremmelgaard A. Analysis of vanillylmandelic and homovanillic acids in urine using solid phase sample preparation and high pressure liquid chromatography with electrochemical detection. Scand J Clin Lab Invest. 1985 Sep;45(5):387–391. doi: 10.3109/00365518509155233. [DOI] [PubMed] [Google Scholar]
- Calil H. M., Lesieur P., Gold P. W., Brown G. M., Zavadil A. P., 3rd, Potter W. Z. Hormonal responses to zimelidine and desipramine in depressed patients. Psychiatry Res. 1984 Nov;13(3):231–242. doi: 10.1016/0165-1781(84)90038-6. [DOI] [PubMed] [Google Scholar]
- Casacchia M., Carolei A., Barba C., Frontoni M., Rossi A., Meco G., Zylberman M. R. A placebo-controlled study of the antidepressant activity of moclobemide, a new MAO-A inhibitor. Pharmacopsychiatry. 1984 Jul;17(4):122–125. doi: 10.1055/s-2007-1017421. [DOI] [PubMed] [Google Scholar]
- Charney D. S., Heninger G. R., Reinhard J. F., Jr, Sternberg D. E., Hafstead K. M. The effect of intravenous L-tryptophan on prolactin and growth hormone and mood in healthy subjects. Psychopharmacology (Berl) 1982;77(3):217–222. doi: 10.1007/BF00464569. [DOI] [PubMed] [Google Scholar]
- Charney D. S., Heninger G. R., Sternberg D. E. Serotonin function and mechanism of action of antidepressant treatment. Effects of amitriptyline and desipramine. Arch Gen Psychiatry. 1984 Apr;41(4):359–365. doi: 10.1001/archpsyc.1984.01790150049008. [DOI] [PubMed] [Google Scholar]
- Checkley S. A. Neuroendocrine tests of monoamine function in man: a review of basic theory and its application to the study of depressive illness. Psychol Med. 1980 Feb;10(1):35–53. doi: 10.1017/s0033291700039593. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G., Goldstein D. S., Stull R., Keiser H. R., Sunderland T., Murphy D. L., Kopin I. J. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem. 1986 Nov;32(11):2030–2033. [PubMed] [Google Scholar]
- Gasic S., Korn A., Eichler H. G., Oberhummer I., Zapotoczky H. G. Cardiocirculatory effects of moclobemide (Ro 11-1163), a new reversible, a short-acting MAO-inhibitor with preferential type A inhibition, in healthy volunteers and depressive patients. Eur J Clin Pharmacol. 1983;25(2):173–177. doi: 10.1007/BF00543787. [DOI] [PubMed] [Google Scholar]
- Glover V., Sandler M., Owen F., Riley G. J. Dopamine is a monoamine oxidase B substrate in man. Nature. 1977 Jan 6;265(5589):80–81. doi: 10.1038/265080a0. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Tipton K. F. Multiple forms of monoamine oxidase: fact and artefact. Life Sci. 1976 Aug 15;19(4):467–477. doi: 10.1016/0024-3205(76)90224-1. [DOI] [PubMed] [Google Scholar]
- Izzo J. L., Jr, Thompson D. A., Horwitz D. Plasma dihydroxyphenylglycol (DHPG) in the in vivo assessment of human neuronal norepinephrine metabolism. Life Sci. 1985 Sep 16;37(11):1033–1038. doi: 10.1016/0024-3205(85)90593-4. [DOI] [PubMed] [Google Scholar]
- Jarrott B., Vajda F. J. The current status of monoamine oxidase and its inhibitors. Med J Aust. 1987 Jun 15;146(12):634–638. doi: 10.5694/j.1326-5377.1987.tb120442.x. [DOI] [PubMed] [Google Scholar]
- Johnston J. P. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968 Jul;17(7):1285–1297. doi: 10.1016/0006-2952(68)90066-x. [DOI] [PubMed] [Google Scholar]
- Jouve J., Mariotte N., Sureau C., Muh J. P. High-performance liquid chromatography with electrochemical detection for the simultaneous determination of the methoxylated amines, normetanephrine, metanephrine and 3-methoxytyramine, in urine. J Chromatogr. 1983 May 13;274:53–62. doi: 10.1016/s0378-4347(00)84408-4. [DOI] [PubMed] [Google Scholar]
- Keller H. H., Kettler R., Keller G., Da Prada M. Short-acting novel MAO inhibitors: in vitro evidence for the reversibility of MAO inhibition by moclobemide and Ro 16-6491. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jan;335(1):12–20. doi: 10.1007/BF00165029. [DOI] [PubMed] [Google Scholar]
- Keselman H. J., Keselman J. C. The analysis of repeated measures designs in medical research. Stat Med. 1984 Apr-Jun;3(2):185–195. doi: 10.1002/sim.4780030211. [DOI] [PubMed] [Google Scholar]
- Korn A., Da Prada M., Raffesberg W., Gasic S., Eichler H. G. Effect of moclobemide, a new reversible monoamine oxidase inhibitor, on absorption and pressor effect of tyramine. J Cardiovasc Pharmacol. 1988 Jan;11(1):17–23. doi: 10.1097/00005344-198801000-00003. [DOI] [PubMed] [Google Scholar]
- Laakmann G., Schoen H. W., Blaschke D., Wittmann M. Dose-dependent growth hormone, prolactin and cortisol stimulation after i.v. administration of desimipramine in human subjects. Psychoneuroendocrinology. 1985;10(1):83–93. doi: 10.1016/0306-4530(85)90042-3. [DOI] [PubMed] [Google Scholar]
- Laakmann G., Schoen H. W., Zygan K., Weiss A., Wittmann M., Meissner R. Effects of receptor blockers (methysergide, propranolol, phentolamine, yohimbine and prazosin) on desimipramine-induced pituitary hormone stimulation in humans--II. Prolactin. Psychoneuroendocrinology. 1986;11(4):463–474. doi: 10.1016/0306-4530(86)90007-7. [DOI] [PubMed] [Google Scholar]
- Lancranjan I., Wirz-Justice A., Pühringer W., Del Pozo E. Effect of 1-5 hydroxytryptophan infusion on growth hormone and prolactin secretion in man. J Clin Endocrinol Metab. 1977 Sep;45(3):588–593. doi: 10.1210/jcem-45-3-588. [DOI] [PubMed] [Google Scholar]
- Larsen J. K., Holm P., Mikkelsen P. L. Moclobemide and clomipramine in the treatment of depression. A randomized clinical trial. Acta Psychiatr Scand. 1984 Sep;70(3):254–260. doi: 10.1111/j.1600-0447.1984.tb01206.x. [DOI] [PubMed] [Google Scholar]
- Lensch K., Fuchs G., Böning J., Milech U. A clinical study of the selective MAO-A-inhibitor moclobemide (Ro 11-1163): a comparison of 2 different dosages with particular reference to platelet MAO-activity and urinary MHPG-excretion. Int Clin Psychopharmacol. 1987 Apr;2(2):165–171. doi: 10.1097/00004850-198704000-00011. [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Sherman B. M. Serotonergic regulation of prolactin and growth hormone secretion in man. Acta Endocrinol (Copenh) 1985 Oct;110(2):152–157. doi: 10.1530/acta.0.1100152. [DOI] [PubMed] [Google Scholar]
- Liebowitz M. R., Karoum F., Quitkin F. M., Davies S. O., Schwartz D., Levitt M., Linnoila M. Biochemical effects of L-deprenyl in atypical depressives. Biol Psychiatry. 1985 May;20(5):558–565. doi: 10.1016/0006-3223(85)90027-7. [DOI] [PubMed] [Google Scholar]
- Mann J. J., Aarons S. F., Frances A. J., Brown R. D. Studies of selective and reversible monoamine oxidase inhibitors. J Clin Psychiatry. 1984 Jul;45(7 Pt 2):62–66. [PubMed] [Google Scholar]
- Mueller E. A., Murphy D. L., Sunderland T. Further studies of the putative serotonin agonist, m-chlorophenylpiperazine: evidence for a serotonin receptor mediated mechanism of action in humans. Psychopharmacology (Berl) 1986;89(3):388–391. doi: 10.1007/BF00174380. [DOI] [PubMed] [Google Scholar]
- Murphy D. L., Garrick N. A., Aulakh C. S., Cohen R. M. New contributions from basic science to understanding the effects of monoamine oxidase inhibiting antidepressants. J Clin Psychiatry. 1984 Jul;45(7 Pt 2):37–43. [PubMed] [Google Scholar]
- Norman T. R., Ames D., Burrows G. D., Davies B. A controlled study of a specific MAO A reversible inhibitor (R011-1163) and amitriptyline in depressive illness. J Affect Disord. 1985 Jan-Feb;8(1):29–35. doi: 10.1016/0165-0327(85)90069-2. [DOI] [PubMed] [Google Scholar]
- Nutt D., Middleton H., Franklin M. The neuroendocrine effects of oral imipramine. Psychoneuroendocrinology. 1987;12(5):367–375. doi: 10.1016/0306-4530(87)90065-5. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pickar D., Cohen R. M., Jimerson D. C., Lake C. R., Murphy D. L. Tyramine infusions and selective monoamine oxidase inhibitor treatment. II. Interrelationships among pressor sensitivity changes, platelet MAO inhibition, and plasma MHPG reduction. Psychopharmacology (Berl) 1981;74(1):8–12. doi: 10.1007/BF00431748. [DOI] [PubMed] [Google Scholar]
- Quattrone A., Tedeschi G., Aguglia U., Scopacasa F., Direnzo G. F., Annunziato L. Prolactin secretion in man: a useful tool to evaluate the activity of drugs on central 5-hydroxytryptaminergic neurones. Studies with fenfluramine. Br J Clin Pharmacol. 1983 Nov;16(5):471–475. doi: 10.1111/j.1365-2125.1983.tb02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer P., Youdim M. B. Monoamine oxidase activity and monoamine metabolism in brains of parkinsonian patients treated with l-deprenyl. J Neurochem. 1986 May;46(5):1359–1365. doi: 10.1111/j.1471-4159.1986.tb01747.x. [DOI] [PubMed] [Google Scholar]
- Scheinin M., Koulu M., Laurikainen E., Allonen H. Hypokalaemia and other non-bronchial effects of inhaled fenoterol and salbutamol: a placebo-controlled dose-response study in healthy volunteers. Br J Clin Pharmacol. 1987 Nov;24(5):645–653. doi: 10.1111/j.1365-2125.1987.tb03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater S. L., Lipper S., Shiling D. J., Murphy D. L. Elevation of plasma-prolactin by monoamine-oxidase inhibitors. Lancet. 1977 Aug 6;2(8032):275–276. doi: 10.1016/s0140-6736(77)90956-4. [DOI] [PubMed] [Google Scholar]
- Sunderland T., Tariot P. N., Cohen R. M., Newhouse P. A., Mellow A. M., Mueller E. A., Murphy D. L. Dose-dependent effects of deprenyl on CSF monoamine metabolites in patients with Alzheimer's disease. Psychopharmacology (Berl) 1987;91(3):293–296. doi: 10.1007/BF00518180. [DOI] [PubMed] [Google Scholar]
- Tuomisto J., Männistö P. Neurotransmitter regulation of anterior pituitary hormones. Pharmacol Rev. 1985 Sep;37(3):249–332. [PubMed] [Google Scholar]
- Westerink B. H., Bosker F. J., O'Hanlon J. F. Use of alumina, sephadex G10, and ion-exchange columns to purify samples for determination of epinephrine, norepinephrine, dopamine, homovanillic acid, and 5-hydroxyindoleacetic acid in urine. Clin Chem. 1982 Aug;28(8):1745–1748. [PubMed] [Google Scholar]
- Winokur A., Lindberg N. D., Lucki I., Phillips J., Amsterdam J. D. Hormonal and behavioral effects associated with intravenous L-tryptophan administration. Psychopharmacology (Berl) 1986;88(2):213–219. doi: 10.1007/BF00652243. [DOI] [PubMed] [Google Scholar]


