Abstract
1. Phenytoin protein binding in epileptic patients on phenytoin as monotherapy has been compared with protein binding in patients treated with both phenytoin and sodium valproate. In addition the relative value of assayed total phenytoin plasma concentrations and assayed unbound phenytoin plasma concentrations and the value of predicted unbound phenytoin plasma concentrations in predicting phenytoin toxicity has been assessed. 2. The mean phenytoin unbound fraction for patients taking sodium valproate (0.122) was significantly greater than for those on monotherapy (0.082). 3. There were six episodes of clinical toxicity. In five toxic episodes the assayed unbound phenytoin plasma concentration was a better reflection of toxicity than the assayed total phenytoin plasma concentration, and four of these occurred in patients on sodium valproate. 4. Unbound phenytoin plasma concentrations were predicted from a single regression equation correlating all assayed total phenytoin plasma concentrations with assayed unbound phenytoin plasma concentrations, from two separate regression equations for each group of patients, and from the correlation between phenytoin protein binding and plasma albumin concentration. 5. The unbound phenytoin plasma concentrations predicted from the two regression equations were statistically no less effective than the assayed unbound phenytoin plasma concentrations in assessing toxicity. 6. Despite a correlation between plasma albumin concentrations and phenytoin protein binding, the use of albumin concentrations in predicting unbound phenytoin plasma concentrations appeared to be of little additional benefit.
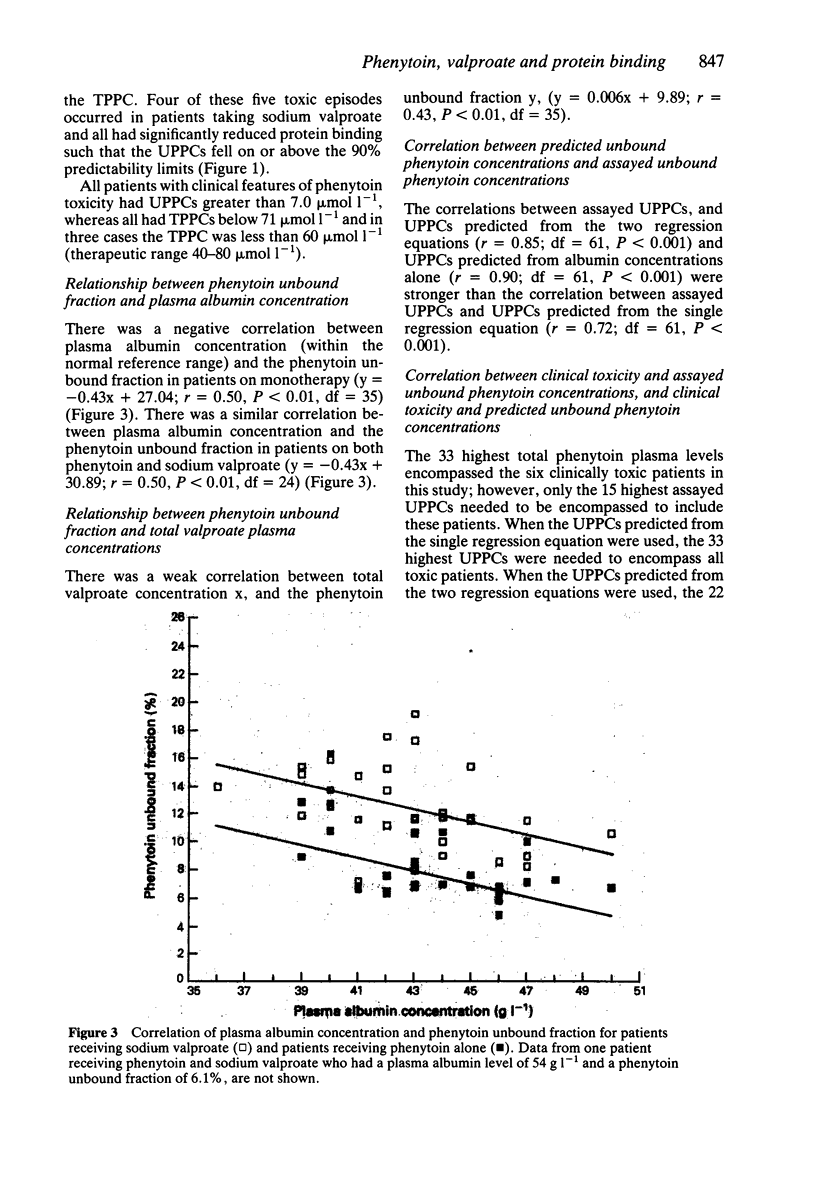
Full text
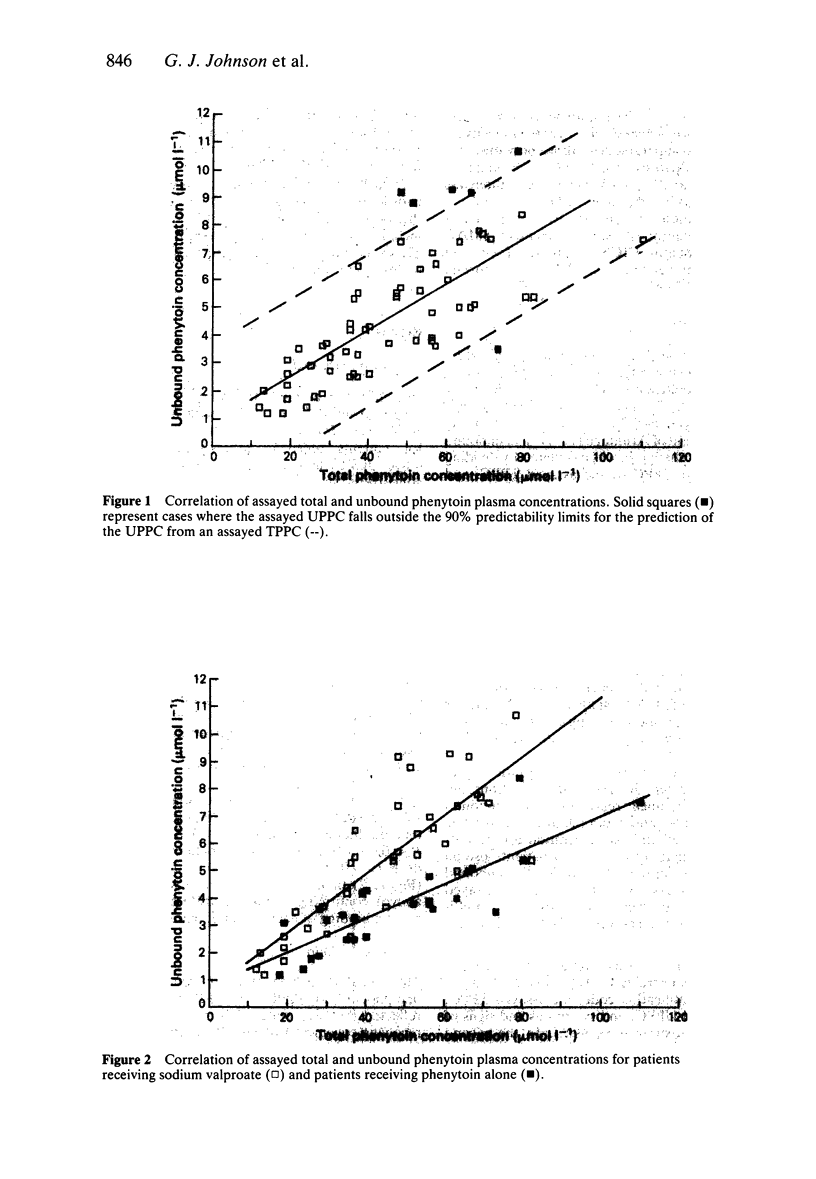
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booker H. E., Darcey B. Serum concentrations of free diphenylhydantoin and their relationship to clinical intoxication. Epilepsia. 1973 Jun;14(2):177–184. doi: 10.1111/j.1528-1157.1973.tb03954.x. [DOI] [PubMed] [Google Scholar]
- DeMonaco H. J., Lawless L. M. Variability of phenytoin protein binding in epileptic patients. Arch Neurol. 1983 Aug;40(8):481–483. doi: 10.1001/archneur.1983.04210070021007. [DOI] [PubMed] [Google Scholar]
- Hooper W. D., Bochner F., Eadie M. J., Tyrer J. H. Plasma protein binding of diphenylhydantoin. Effects of sex hormones, renal and hepatic disease. Clin Pharmacol Ther. 1974 Mar;15(3):276–282. doi: 10.1002/cpt1974153276. [DOI] [PubMed] [Google Scholar]
- KUTT H., WINTERS W., KOKENGE R., MCDOWELL F. DIPHENYLHYDANTOIN METABOLISM, BLOOD LEVELS, AND TOXICITY. Arch Neurol. 1964 Dec;11:642–648. doi: 10.1001/archneur.1964.00460240074010. [DOI] [PubMed] [Google Scholar]
- Kilpatrick C. J., Wanwimolruk S., Wing L. M. Plasma concentrations of unbound phenytoin in the management of epilepsy. Br J Clin Pharmacol. 1984 May;17(5):539–546. doi: 10.1111/j.1365-2125.1984.tb02387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott C., Hamshaw-Thomas A., Reynolds F. Phenytoin-valproate interaction: importance of saliva monitoring in epilepsy. Br Med J (Clin Res Ed) 1982 Jan 2;284(6308):13–16. doi: 10.1136/bmj.284.6308.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. H., Moreland T. A. Rationale for monitoring free drug levels. Clin Pharmacokinet. 1984 Jan;9 (Suppl 1):1–9. doi: 10.2165/00003088-198400091-00001. [DOI] [PubMed] [Google Scholar]
- Lund L. Anticonvulsant effect of diphenylhydantoin relative to plasma levels. A prospective three-year study in ambulant patients with generalized epileptic seizures. Arch Neurol. 1974 Nov;31(5):289–294. doi: 10.1001/archneur.1974.00490410037002. [DOI] [PubMed] [Google Scholar]
- McAuliffe J. J., Sherwin A. L., Leppik I. E., Fayle S. A., Pippenger C. E. Salivary levels of anticonvulsants: a practical approach to drug monitoring. Neurology. 1977 May;27(5):409–413. doi: 10.1212/wnl.27.5.409. [DOI] [PubMed] [Google Scholar]
- Perucca E. Free level monitoring of antiepileptic drugs. Clinical usefulness and case studies. Clin Pharmacokinet. 1984 Jan;9 (Suppl 1):71–78. doi: 10.2165/00003088-198400091-00009. [DOI] [PubMed] [Google Scholar]
- Perucca E., Hebdige S., Frigo G. M., Gatti G., Lecchini S., Crema A. Interaction between phenytoin and valproic acid: plasma protein binding and metabolic effects. Clin Pharmacol Ther. 1980 Dec;28(6):779–789. doi: 10.1038/clpt.1980.235. [DOI] [PubMed] [Google Scholar]
- Porter R. J., Layzer R. B. Plasma albumin concentration and diphenylhydantoin binding in man. Arch Neurol. 1975 May;32(5):298–303. doi: 10.1001/archneur.1975.00490470042005. [DOI] [PubMed] [Google Scholar]
- Reynolds F., Ziroyanis P. N., Jones N. F., Smith S. E. Salivary phenytoin concentrations in epilepsy and in chronic renal failure. Lancet. 1976 Aug 21;2(7982):384–386. doi: 10.1016/s0140-6736(76)92404-1. [DOI] [PubMed] [Google Scholar]
- Tsanaclis L. M., Allen J., Perucca E., Routledge P. A., Richens A. Effect of valproate on free plasma phenytoin concentrations. Br J Clin Pharmacol. 1984 Jul;18(1):17–20. doi: 10.1111/j.1365-2125.1984.tb05015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


