Abstract
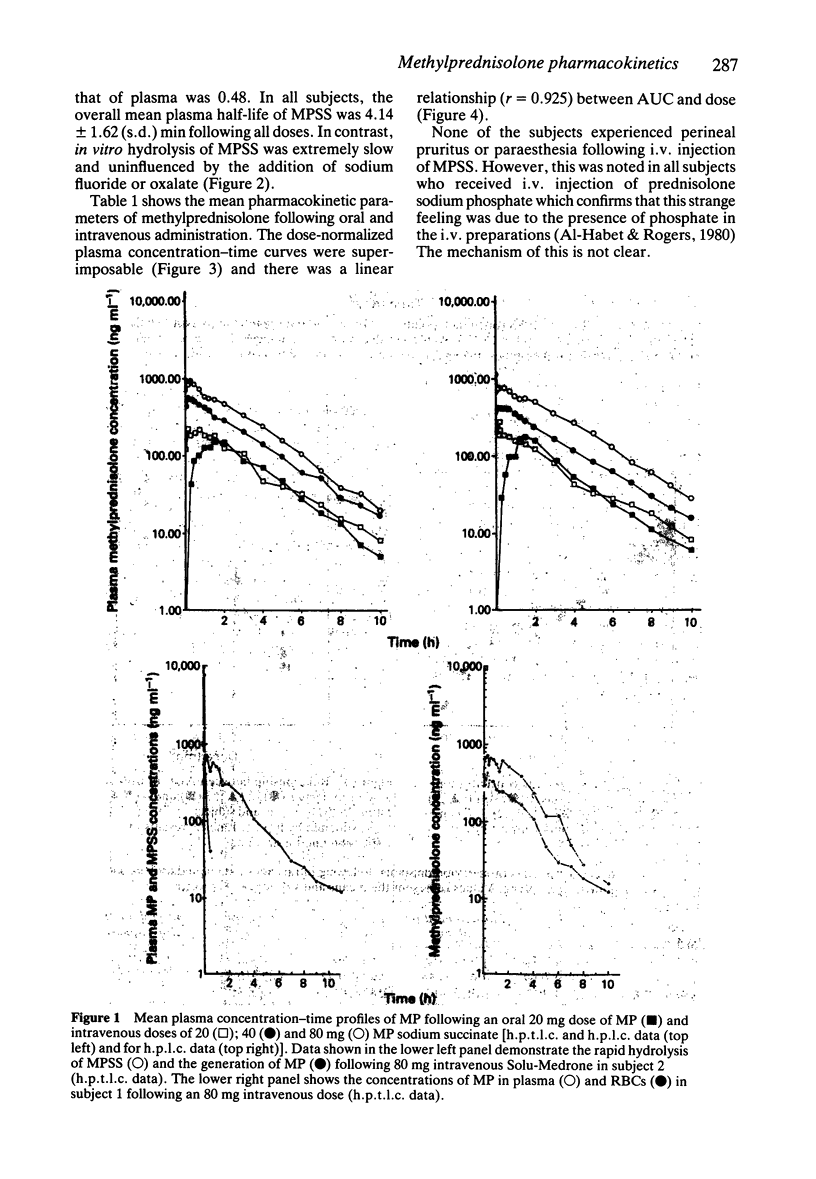
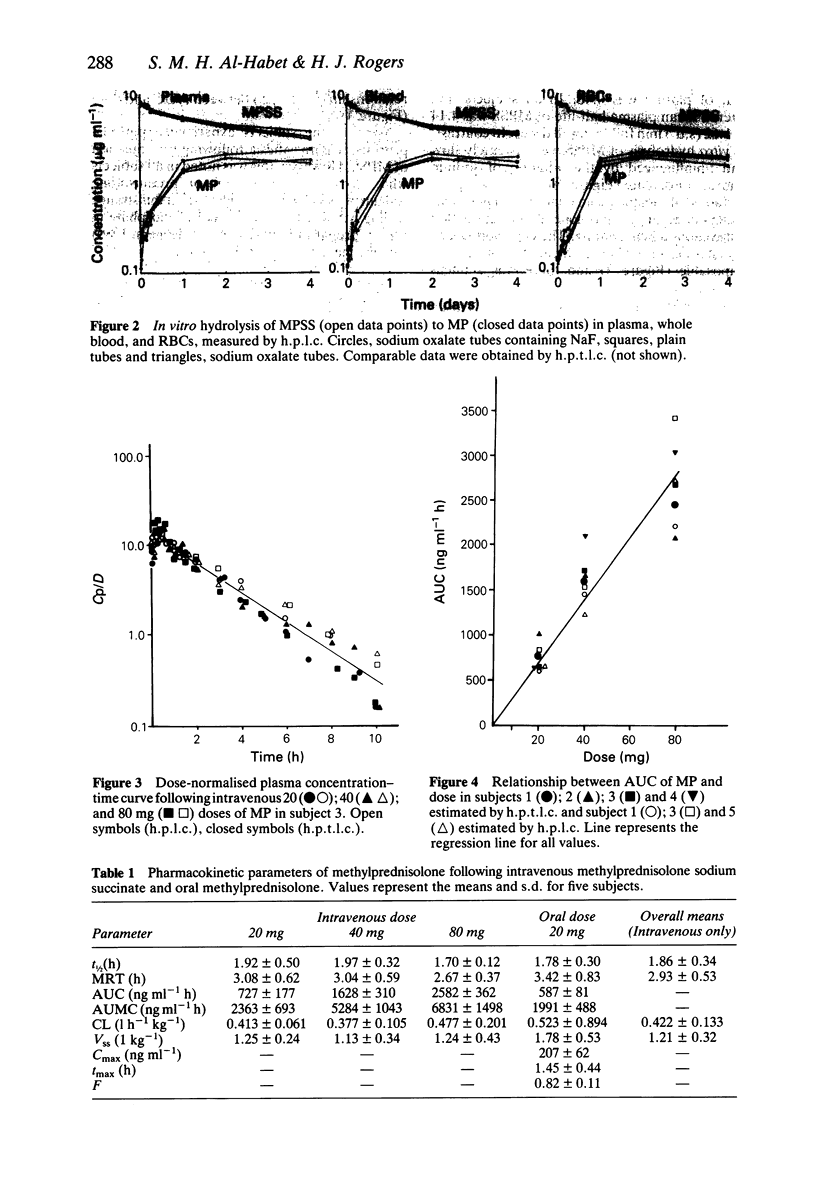
1. The pharmacokinetics of methylprednisolone (MP) were studied in five normal subjects following intravenous doses of 20, 40 and 80 mg methylprednisolone sodium succinate (MPSS) and an oral dose of 20 mg methylprednisolone as 4 x 5 mg tablets. Plasma concentrations of MP and MPSS were measured by both high performance thin layer (h.p.t.l.c.) and high pressure liquid chromatography (h.p.l.c.). 2. The mean values (+/- s.d.) of half-life, mean residence time (MRT), systemic clearance (CL) and volume of distribution at steady state (Vss) of MP following intravenous administration were 1.93 +/- 0.35 h, 3.50 +/- 1.01 h, 0.45 +/- 0.12 lh-1 kg-1 and 1.5 +/- 0.63 1 kg-1, respectively. There was no evidence of dose-related changes in these values. The plasma MP concentration-time curves were superimposable when normalized for dose. 3. The bioavailability of methylprednisolone from the 20 mg tablet was 0.82 +/- 0.11 (s.d.). 4. In vivo hydrolysis of MPSS was rapid with a half-life of 4.14 +/- 1.62 (s.d.) min, and was independent of dose. In contrast, in vitro hydrolysis in plasma, whole blood and red blood cells was slow; the process continuing for more than 7 days. Sodium fluoride did not prevent the hydrolysis of MPSS.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Habet S. M., McAllister W. A., Collins J. V., Rogers H. J. Comparison of radioimmunoassay and thin layer chromatographic assay methods for estimation of plasma prednisolone concentrations. J Pharmacol Methods. 1981 Sep;6(2):137–142. doi: 10.1016/0160-5402(81)90036-x. [DOI] [PubMed] [Google Scholar]
- Al-Habet S. M., Rogers H. J. Prednisolone elimination in human saliva. Int J Clin Pharmacol Ther Toxicol. 1985 Sep;23(9):485–487. [PubMed] [Google Scholar]
- Al-Habet S., Rogers H. J. Pharmacokinetics of intravenous and oral prednisolone. Br J Clin Pharmacol. 1980 Nov;10(5):503–508. doi: 10.1111/j.1365-2125.1980.tb01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert K. S., Brown S. W., Jr, DeSante K. A., DiSanto A. R., Stewart R. D., Chen T. T. Double Latin square study to determine variability and relative bioavailability of methylprednisolone. J Pharm Sci. 1979 Oct;68(10):1312–1316. doi: 10.1002/jps.2600681031. [DOI] [PubMed] [Google Scholar]
- Anderson B. D., Conradi R. A., Knuth K. E. Strategies in the design of solution-stable, water-soluble prodrugs I: a physical-organic approach to pro-moiety selection for 21-esters of corticosteroids. J Pharm Sci. 1985 Apr;74(4):365–374. doi: 10.1002/jps.2600740402. [DOI] [PubMed] [Google Scholar]
- Antal E. J., Wright C. E., 3rd, Gillespie W. R., Albert K. S. Influence of route of administration on the pharmacokinetics of methylprednisolone. J Pharmacokinet Biopharm. 1983 Dec;11(6):561–576. doi: 10.1007/BF01059057. [DOI] [PubMed] [Google Scholar]
- Assael B. M., Banfi G., Appiani A. C., Edefonti A., Jusko W. J. Disposition of pulse dose methylprednisolone in adult and paediatric patients with the nephrotic syndrome. Eur J Clin Pharmacol. 1982;23(5):429–433. doi: 10.1007/BF00605993. [DOI] [PubMed] [Google Scholar]
- Baylis E. M., Williams I. A., English J., Marks V., Chakraborty J. High dose intravenous methylprednisolone "pulse" therapy in patients with rheumatoid disease. Plasma methylprednisolone levels and adrenal function. Eur J Clin Pharmacol. 1982;21(5):385–388. doi: 10.1007/BF00542323. [DOI] [PubMed] [Google Scholar]
- Brady M. E., Sartiano G. P., Rosenblum S. L., Zaglama N. E., Bauguess C. T. The pharmacokinetics of single high doses of dexamethasone in cancer patients. Eur J Clin Pharmacol. 1987;32(6):593–596. doi: 10.1007/BF02455994. [DOI] [PubMed] [Google Scholar]
- Cutler D. J. Definition of mean residence times in pharmacokinetics. Biopharm Drug Dispos. 1987 Jan-Feb;8(1):87–97. doi: 10.1002/bdd.2510080110. [DOI] [PubMed] [Google Scholar]
- Derendorf H., Möllmann H., Rohdewald P., Rehder J., Schmidt E. W. Kinetics of methylprednisolone and its hemisuccinate ester. Clin Pharmacol Ther. 1985 May;37(5):502–507. doi: 10.1038/clpt.1985.79. [DOI] [PubMed] [Google Scholar]
- Ebling W. F., Szefler S. J., Jusko W. J. Analysis of cortisol, methylprednisolone, and methylprednisolone hemisuccinate. Absence of effects of troleandomycin on ester hydrolysis. J Chromatogr. 1984 Feb 10;305(2):271–280. [PubMed] [Google Scholar]
- Ebling W. F., Szefler S. J., Jusko W. J. Methylprednisolone disposition in rabbits. Analysis, prodrug conversion, reversible metabolism, and comparison with man. Drug Metab Dispos. 1985 May-Jun;13(3):296–304. [PubMed] [Google Scholar]
- Ferry J. J., Wagner J. G. The nonlinear pharmacokinetics of prednisone and prednisolone. II. Plasma protein binding of prednisone and prednisolone in rabbit and human plasma. Biopharm Drug Dispos. 1987 May-Jun;8(3):261–272. doi: 10.1002/bdd.2510080307. [DOI] [PubMed] [Google Scholar]
- Garg D. C., Ayres J. W., Wagner J. G. Determination of methylprednisolone and hydrocortisone in plasma using high pressure liquid chromatography. Res Commun Chem Pathol Pharmacol. 1977 Sep;18(1):137–146. [PubMed] [Google Scholar]
- Garg D. C., Ng P., Weidler D. J., Sakmar E., Wagner J. G. Preliminary in vitro and in vivo investigations on methylprednisolone and its acetate. Res Commun Chem Pathol Pharmacol. 1978 Oct;22(1):37–48. [PubMed] [Google Scholar]
- Garg D. C., Wagner J. G., Sakmar E., Weidler D. J., Albert K. S. Rectal and oral absorption of methylprednisolone acetate. Clin Pharmacol Ther. 1979 Aug;26(2):232–239. doi: 10.1002/cpt1979262232. [DOI] [PubMed] [Google Scholar]
- Kong A. N., Jusko W. J. Definitions and applications of mean transit and residence times in reference to the two-compartment mammillary plasma clearance model. J Pharm Sci. 1988 Feb;77(2):157–165. doi: 10.1002/jps.2600770213. [DOI] [PubMed] [Google Scholar]
- Loew D., Schuster O., Graul E. H. Dose-dependent pharmacokinetics of dexamethasone. Eur J Clin Pharmacol. 1986;30(2):225–230. doi: 10.1007/BF00614309. [DOI] [PubMed] [Google Scholar]
- Meyers C., Lockridge O., La Du B. N. Hydrolysis of methylprednisolone acetate by human serum cholinesterase. Drug Metab Dispos. 1982 May-Jun;10(3):279–280. [PubMed] [Google Scholar]
- Narang P. K., Wilder R., Chatterji D. C., Yeager R. L., Gallelli J. F. Systemic bioavailability and pharmacokinetics of methylprednisolone in patients with rheumatoid arthritis following 'high-dose' pulse administration. Biopharm Drug Dispos. 1983 Jul-Sep;4(3):233–248. doi: 10.1002/bdd.2510040305. [DOI] [PubMed] [Google Scholar]
- Rohdewald P., Möllmann H., Barth J., Rehder J., Derendorf H. Pharmacokinetics of dexamethasone and its phosphate ester. Biopharm Drug Dispos. 1987 May-Jun;8(3):205–212. doi: 10.1002/bdd.2510080302. [DOI] [PubMed] [Google Scholar]
- Rose J. Q., Yurchak A. M., Jusko W. J. Dose dependent pharmacokinetics of prednisone and prednisolone in man. J Pharmacokinet Biopharm. 1981 Aug;9(4):389–417. doi: 10.1007/BF01060885. [DOI] [PubMed] [Google Scholar]
- Shah J. A., Weber D. J., Bothwell B. E. High-performance liquid chromatographic determination of methylprednisolone and methylprednisolone 21-[8-[methyl-(2-sulfoethyl)amino]-8-oxoctanoate] sodium salt in human plasma. J Chromatogr. 1987 Feb 20;414(1):1–10. doi: 10.1016/0378-4347(87)80018-x. [DOI] [PubMed] [Google Scholar]
- Shah J. A., Weber D. J. High-performance liquid chromatographic assay for methylprednisolone and its soluble prodrug esters in dog plasma. J Chromatogr. 1985 Nov 8;344:41–49. doi: 10.1016/s0378-4347(00)82005-8. [DOI] [PubMed] [Google Scholar]
- Szefler S. J., Ebling W. F., Georgitis J. W., Jusko W. J. Methylprednisolone versus prednisolone pharmacokinetics in relation to dose in adults. Eur J Clin Pharmacol. 1986;30(3):323–329. doi: 10.1007/BF00541537. [DOI] [PubMed] [Google Scholar]
- Tanner A., Bochner F., Caffin J., Halliday J., Powell L. Dose-dependent prednisolone kinetics. Clin Pharmacol Ther. 1979 May;25(5 Pt 1):571–578. doi: 10.1002/cpt1979255part1571. [DOI] [PubMed] [Google Scholar]


