Abstract
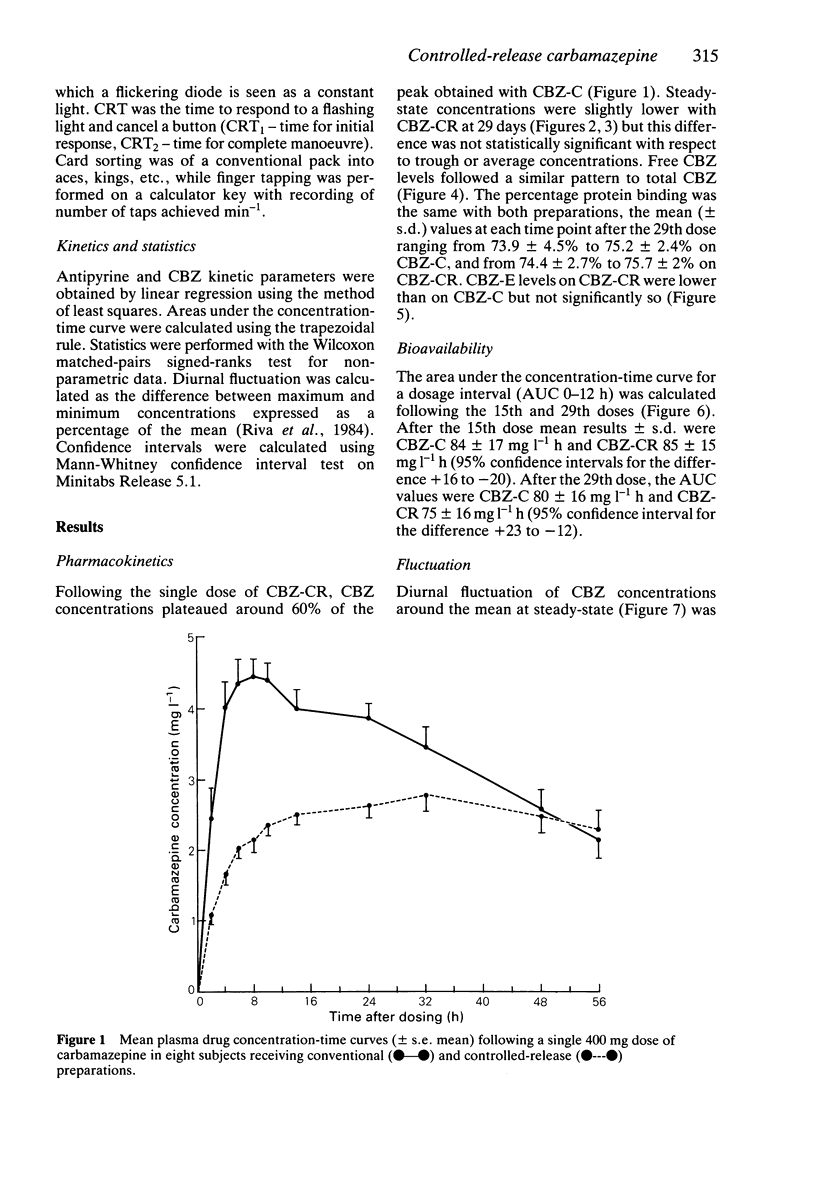
1. Eight healthy subjects took part in a balanced, double-blind, crossover comparison of conventional carbamazepine (Tegretol, Ciba-Geigy Ltd, CBZ-C) and a novel controlled-release formulation (Tegretol CR Divitabs, Ciba-Geigy Ltd; CBZ-CR). An initial single dose of either preparation was followed 1 week later by a 2 week course of 200 mg twice daily. 2. Following the single dose, CBZ-CR produced a concentration plateau from 6-56 h at 50-60% of the CBZ-CR peak. 3. After 2 weeks' treatment, CBZ daytime levels measured as area under the concentration-time curve over a dosage interval were 7% lower with CBZ-CR, but this difference was not statistically significant. 4. CBZ-CR showed less diurnal fluctuation (12%) of CBZ than CBZ-C (24%; P less than 0.025) with less rapid changes in concentration (P less than 0.02). 5. Diurnal fluctuation of free CBZ and of CBZ 10,11 epoxide, the active metabolite, did not differ significantly between the two preparations. 6. Auto-induction of CBZ metabolism resulted from the administration of both formulations. The mean elimination half-life was 23 h (CBZ-C) and 25 h (CBZ-CR) after dose 29 compared with a base-line value of 37 h (both P less than 0.02). Antipyrine metabolism was also induced to a similar extent in both legs of the study (P less than 0.01). 7. No significant alteration in psychomotor function was demonstrated with either preparation. 8. CBZ-CR fulfils the criteria for a controlled-release preparation with comparable apparent bioavailability to CBZ-C. Further pharmacokinetic and, more importantly, pharmacodynamic studies are required in epileptic patients to confirm a clinical advantage over the currently available formulation.
Full text
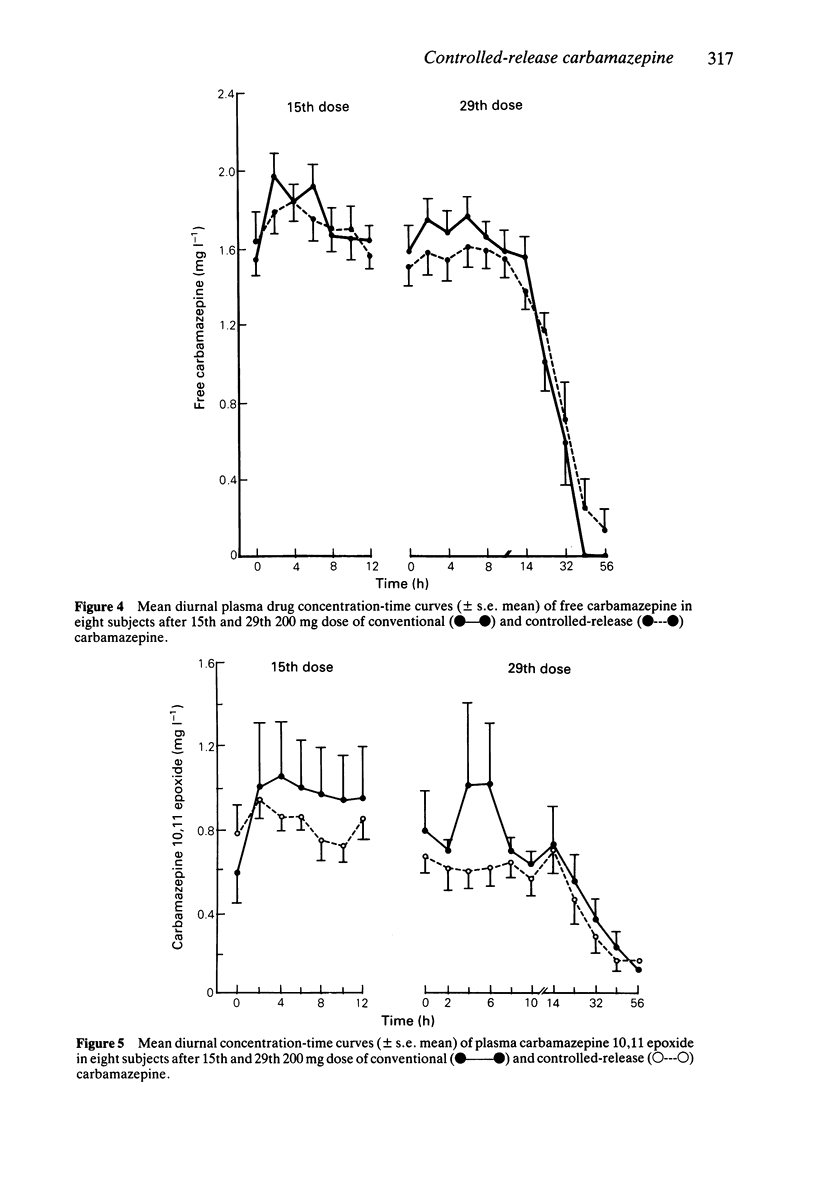
PDF

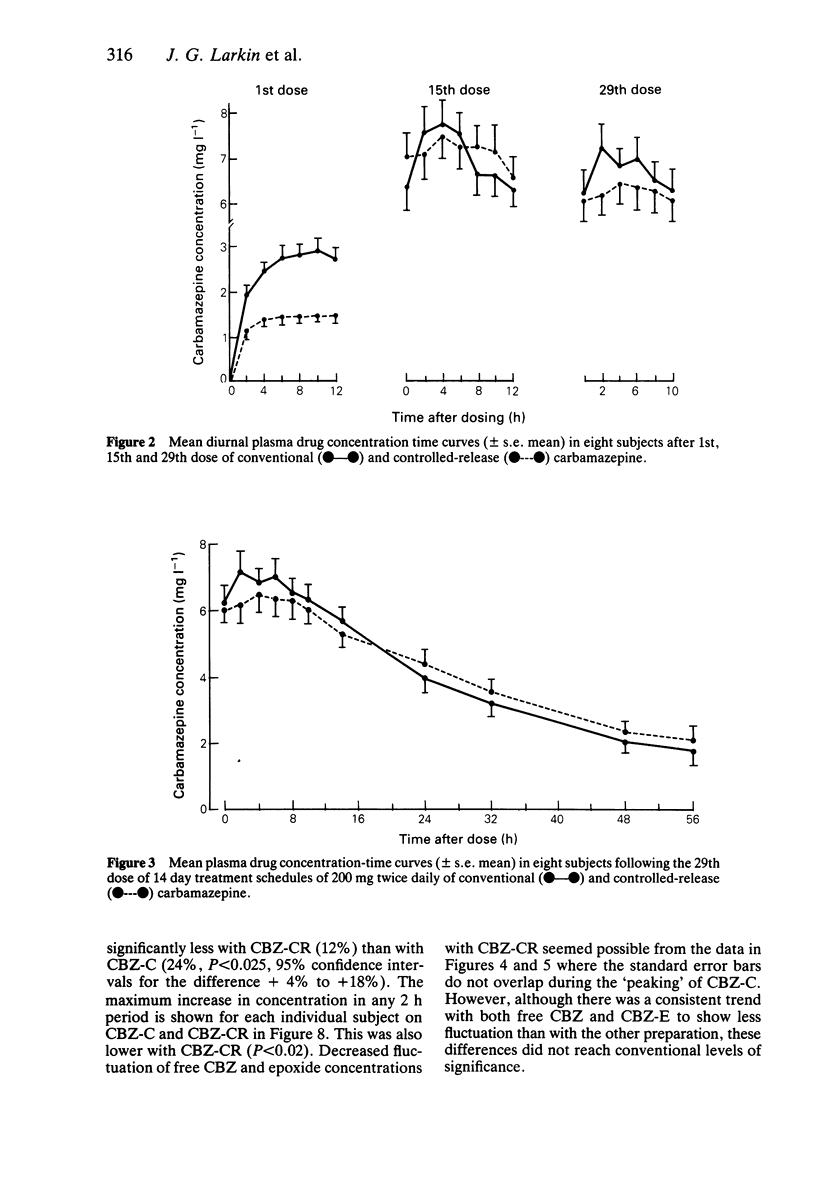
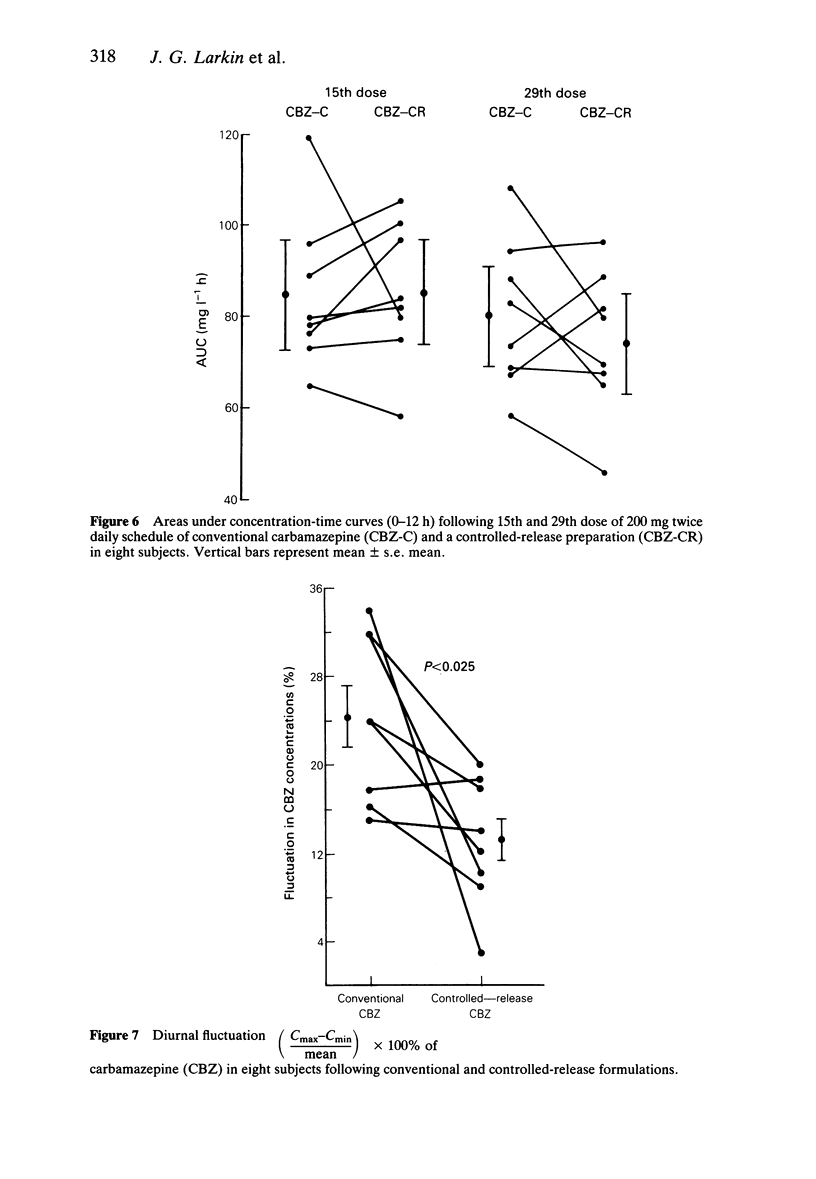
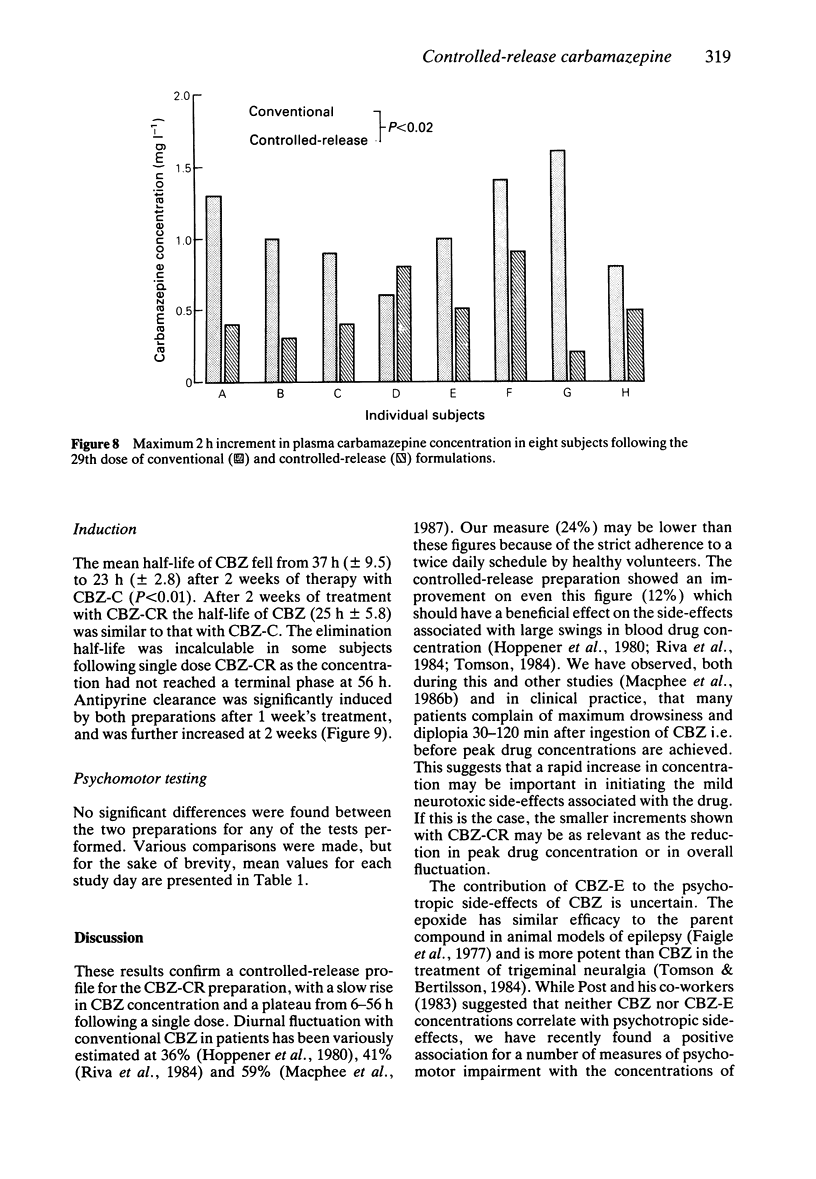
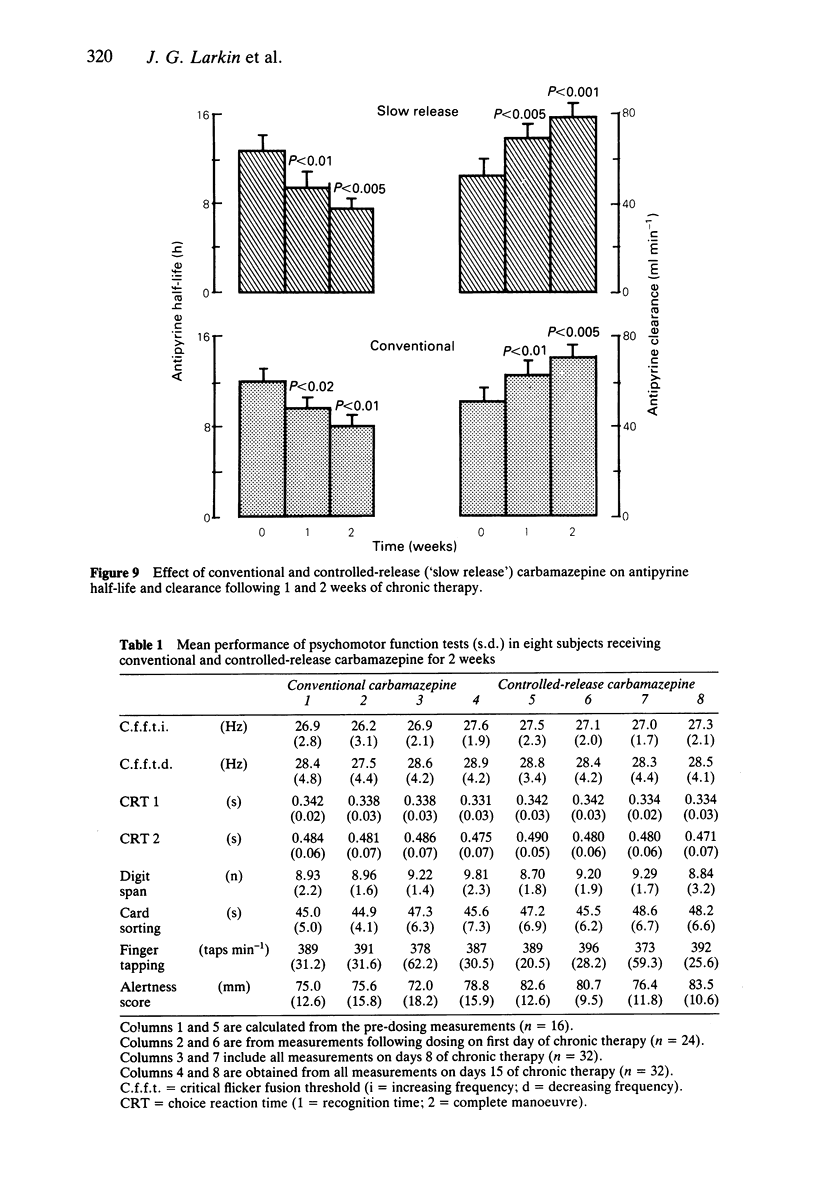


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldenkamp A. P., Alpherts W. C., Moerland M. C., Ottevanger N., Van Parys J. A. Controlled release carbamazepine: cognitive side effects in patients with epilepsy. Epilepsia. 1987 Sep-Oct;28(5):507–514. doi: 10.1111/j.1528-1157.1987.tb03679.x. [DOI] [PubMed] [Google Scholar]
- Brodie M. J., McPhail E., Macphee G. J., Larkin J. G., Gray J. M. Psychomotor impairment and anticonvulsant therapy in adult epileptic patients. Eur J Clin Pharmacol. 1987;31(6):655–660. doi: 10.1007/BF00541291. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Ekbom K., Bertilsson L., Ringberger V. A., Rane A. Plasma kinetics of carbamazepine and its epoxide metabolite in man after single and multiple doses. Eur J Clin Pharmacol. 1975 Jun 13;8(5):337–341. doi: 10.1007/BF00562659. [DOI] [PubMed] [Google Scholar]
- Evans R. W., Gualtieri C. T. Carbamazepine: a neuropsychological and psychiatric profile. Clin Neuropharmacol. 1985;8(3):221–241. [PubMed] [Google Scholar]
- Gillham R. A., Williams N., Wiedmann K., Butler E., Larkin J. G., Brodie M. J. Concentration-effect relationships with carbamazepine and its epoxide on psychomotor and cognitive function in epileptic patients. J Neurol Neurosurg Psychiatry. 1988 Jul;51(7):929–933. doi: 10.1136/jnnp.51.7.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarch I. Psychomotor function and psychoactive drugs. Br J Clin Pharmacol. 1980 Sep;10(3):189–209. doi: 10.1111/j.1365-2125.1980.tb01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höppener R. J., Kuyer A., Meijer J. W., Hulsman J. Correlation between daily fluctuations of carbamazepine serum levels and intermittent side effects. Epilepsia. 1980 Aug;21(4):341–350. doi: 10.1111/j.1528-1157.1980.tb04081.x. [DOI] [PubMed] [Google Scholar]
- Lesser R. P., Pippenger C. E., Lüders H., Dinner D. S. High-dose monotherapy in treatment of intractable seizures. Neurology. 1984 Jun;34(6):707–711. doi: 10.1212/wnl.34.6.707. [DOI] [PubMed] [Google Scholar]
- MacPhee G. J., Goldie C., Roulston D., Potter L., Agnew E., Laidlaw J., Brodie M. J. Effect of carbamazepine on psychomotor performance in näive subjects. Eur J Clin Pharmacol. 1986;30(1):37–42. doi: 10.1007/BF00614193. [DOI] [PubMed] [Google Scholar]
- Macphee G. J., Brodie M. J. Carbamazepine substitution in severe partial epilepsy: implication of autoinduction of metabolism. Postgrad Med J. 1985 Sep;61(719):779–783. doi: 10.1136/pgmj.61.719.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macphee G. J., Butler E., Brodie M. J. Intradose and circadian variation in circulating carbamazepine and its epoxide in epileptic patients: a consequence of autoinduction of metabolism. Epilepsia. 1987 May-Jun;28(3):286–294. doi: 10.1111/j.1528-1157.1987.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Macphee G. J., McPhail E. M., Butler E., Brodie M. J. Controlled evaluation of a supplementary dose of carbamazepine on psychomotor function in epileptic patients. Eur J Clin Pharmacol. 1986;31(2):195–199. doi: 10.1007/BF00606658. [DOI] [PubMed] [Google Scholar]
- Macphee G. J., Thompson G. G., Scobie G., Agnew E., Park B. K., Murray T., McColl K. E., Brodie M. J. Effects of cimetidine on carbamazepine auto- and hetero-induction in man. Br J Clin Pharmacol. 1984 Sep;18(3):411–419. doi: 10.1111/j.1365-2125.1984.tb02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsalos P. N., Stephenson T. J., Krishna S., Elyas A. A., Lascelles P. T., Wiles C. M. Side-effects induced by carbamazepine-10,11-epoxide. Lancet. 1985 Aug 31;2(8453):496–496. doi: 10.1016/s0140-6736(85)90420-9. [DOI] [PubMed] [Google Scholar]
- Post R. M., Uhde T. W., Ballenger J. C., Chatterji D. C., Greene R. F., Bunney W. E., Jr Carbamazepine and its -10,11-epoxide metabolite in plasma and CSF. Relationship to antidepressant response. Arch Gen Psychiatry. 1983 Jun;40(6):673–676. doi: 10.1001/archpsyc.1983.04390010083010. [DOI] [PubMed] [Google Scholar]
- Rapeport W. G., McInnes G. T., Thompson G. G., Forrest G., Park B. K., Brodie M. J. Hepatic enzyme induction and leucocyte delta-aminolaevulinic acid synthase activity: studies with carbamazepine. Br J Clin Pharmacol. 1983 Aug;16(2):133–137. doi: 10.1111/j.1365-2125.1983.tb04976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds E. H. Mental effects of antiepileptic medication: a review. Epilepsia. 1983;24 (Suppl 2):S85–S95. doi: 10.1111/j.1528-1157.1983.tb04651.x. [DOI] [PubMed] [Google Scholar]
- Riva R., Albani F., Ambrosetto G., Contin M., Cortelli P., Perucca E., Baruzzi A. Diurnal fluctuations in free and total steady-state plasma levels of carbamazepine and correlation with intermittent side effects. Epilepsia. 1984 Aug;25(4):476–481. doi: 10.1111/j.1528-1157.1984.tb03446.x. [DOI] [PubMed] [Google Scholar]
- Shargel L., Cheung W. M., Yu A. B. High-pressure liquid chromatographic analysis of antipyrine in small plasma samples. J Pharm Sci. 1979 Aug;68(8):1052–1054. doi: 10.1002/jps.2600680835. [DOI] [PubMed] [Google Scholar]
- Thompson P. J., Trimble M. R. Anticonvulsant drugs and cognitive functions. Epilepsia. 1982 Oct;23(5):531–544. doi: 10.1111/j.1528-1157.1982.tb05439.x. [DOI] [PubMed] [Google Scholar]
- Tomson T., Bertilsson L. Potent therapeutic effect of carbamazepine-10,11-epoxide in trigeminal neuralgia. Arch Neurol. 1984 Jun;41(6):598–601. doi: 10.1001/archneur.1984.04210080006004. [DOI] [PubMed] [Google Scholar]
- Tomson T. Interdosage fluctuations in plasma carbamazepine concentration determine intermittent side effects. Arch Neurol. 1984 Aug;41(8):830–834. doi: 10.1001/archneur.1984.04050190036011. [DOI] [PubMed] [Google Scholar]


