Abstract
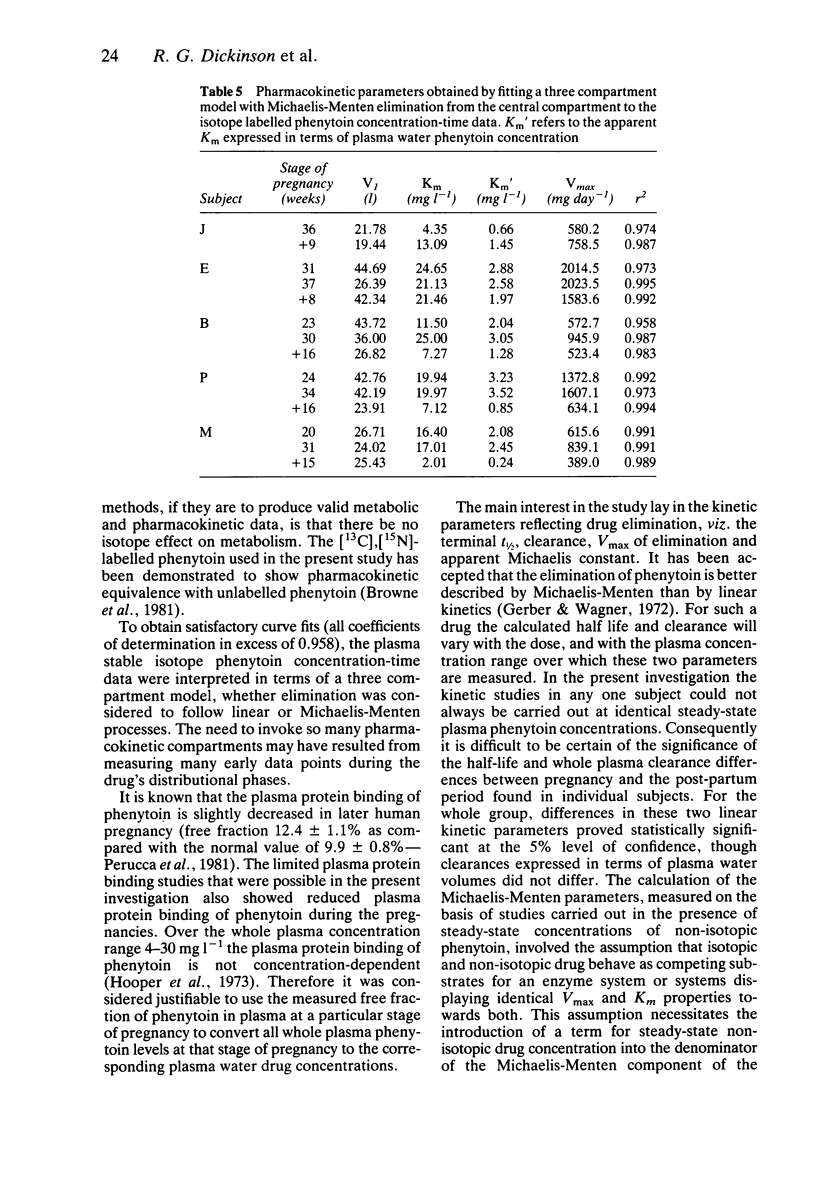
1. To investigate the mechanism of the fall in steady-state plasma phenytoin concentration relative to drug dose that occurs during pregnancy, single dose pharmacokinetic studies with stable isotope labelled phenytoin were carried out at different stages of pregnancy, and 2 to 4 months post-natally, in five epileptic women receiving regular oral therapy with the drug. 2. Steady-state apparent plasma clearances of phenytoin (dose/steady-state concentration) correlated closely with simultaneous plasma clearances of the intravenous stable-isotope drug (measured as dose/AUC) suggesting that the patients were complaint with therapy when their phenytoin dosage requirement increased during the pregnancy, and that the oral drug was fully bioavailable. 3. In retrospect, two of the five subjects were probably studied too early post-natally for phenytoin elimination kinetics to have returned to non-pregnant values. Despite this, (i) the mean +/- s.d. t 1/2 for phenytoin was statistically significantly shorter in pregnancy than post-natally (31 +/- 14 vs 39 +/- 28 h), (ii) the mean +/- s.d. whole plasma clearance was also statistically significant greater (0.025 +/- 0.012 vs 0.021 +/- 0.013 kg-1 h-1) and (iii) the mean +/- s.d. Vmax for phenytoin elimination was statistically significantly greater in pregnancy (1170 +/- 600 mg day-1) than post-natally (780 +/- 470 mg day-1). Although the mean +/- s.d. apparent Km was higher in pregnancy (18.2 +/- 8.4 mg l-1, expressed in terms of whole plasma drug concentrations, compared with 10.2 +/- 7.4 mg l-1 post-natally), the difference was not statistically significant. However, if the apparent Km value was expressed in terms of plasma water phenytoin concentrations the difference (pregnant 2.50 +/- 0.85 mg l-1: post-natally 1.16 +/- 0.65 mg l-1) was statistically significant. 4. Human pregnancy appears to result in an increased capacity to eliminate phenytoin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg M. J., Fischer L. J., Rivey M. P., Vern B. A., Lantz R. K., Schottelius D. D. Phenytoin and folic acid interaction: a preliminary report. Ther Drug Monit. 1983;5(4):389–394. doi: 10.1097/00007691-198312000-00002. [DOI] [PubMed] [Google Scholar]
- Browne T. R., Greenblatt D. J., Harmatz J. S., Evans J. E., Szabo G. K., Evans B. A., Schumacher G. E. Studies with stable isotopes III: Pharmacokinetics of tracer doses of drug. J Clin Pharmacol. 1985 Jan-Feb;25(1):59–63. doi: 10.1002/j.1552-4604.1985.tb02801.x. [DOI] [PubMed] [Google Scholar]
- Browne T. R., Van Langenhove A., Costello C. E., Biemann K., Greenblatt D. J. Kinetic equivalence of stable-isotope-labeled and unlabeled phenytoin. Clin Pharmacol Ther. 1981 Apr;29(4):511–515. doi: 10.1038/clpt.1981.71. [DOI] [PubMed] [Google Scholar]
- Eadie M. J., Lander C. M., Tyrer J. H. Plasma drug level monitoring in pregnancy. Clin Pharmacokinet. 1977 Nov-Dec;2(6):427–436. doi: 10.2165/00003088-197702060-00003. [DOI] [PubMed] [Google Scholar]
- Gerber N., Wagner J. G. Explanation of dose-dependent decline of diphenylhydantoin plasma levels by fitting to the integrated form of the Michaelis-Menten equation. Res Commun Chem Pathol Pharmacol. 1972 May;3(3):455–466. [PubMed] [Google Scholar]
- Glazko A. J., Chang T., Baukema J., Dill W. A., Goulet J. R., Buchanan R. A. Metabolic disposition of diphenylhydantoin in normal human subjects following intravenous administration. Clin Pharmacol Ther. 1969 Jul-Aug;10(4):498–504. doi: 10.1002/cpt1969104498. [DOI] [PubMed] [Google Scholar]
- Hooper W. D., Sutherland J. M., Bochner F., Tyrer J. H., Eadie M. J. The effect of certain drugs on the plasma protein binding of phenytoin. Aust N Z J Med. 1973 Aug;3(4):377–381. doi: 10.1111/j.1445-5994.1973.tb03109.x. [DOI] [PubMed] [Google Scholar]
- Jung D., Powell J. R., Walson P., Perrier D. Effect of dose on phenytoin absorption. Clin Pharmacol Ther. 1980 Oct;28(4):479–485. doi: 10.1038/clpt.1980.191. [DOI] [PubMed] [Google Scholar]
- Kluck R. M., Cannell G. R., Hooper W. D., Eadie M. J., Dickinson R. G. Disposition of phenytoin and phenobarbitone in the isolated perfused human placenta. Clin Exp Pharmacol Physiol. 1988 Nov;15(11):827–836. doi: 10.1111/j.1440-1681.1988.tb01025.x. [DOI] [PubMed] [Google Scholar]
- Knight A. H., Rhind E. G. Epilepsy and pregnancy: a study of 153 pregnancies in 59 patients. Epilepsia. 1975 Mar;16(1):99–110. doi: 10.1111/j.1528-1157.1975.tb04726.x. [DOI] [PubMed] [Google Scholar]
- Kochenour N. K., Emery M. G., Sawchuk R. J. Phenytoin metabolism in pregnancy. Obstet Gynecol. 1980 Nov;56(5):577–582. [PubMed] [Google Scholar]
- Lander C. M., Edwards V. E., Eadie M. J., Tyrer J. H. Plasma anticonvulsant concentrations during pregnancy. Neurology. 1977 Feb;27(2):128–131. doi: 10.1212/wnl.27.2.128. [DOI] [PubMed] [Google Scholar]
- Lander C. M., Smith M. T., Chalk J. B., de Wytt C., Symoniw P., Livingstone I., Eadie M. J. Bioavailability and pharmacokinetics of phenytoin during pregnancy. Eur J Clin Pharmacol. 1984;27(1):105–110. [PubMed] [Google Scholar]
- Landon M. J., Kirkley M. Metabolism of diphenylhydantoin (phenytoin) during pregnancy. Br J Obstet Gynaecol. 1979 Feb;86(2):125–132. doi: 10.1111/j.1471-0528.1979.tb10579.x. [DOI] [PubMed] [Google Scholar]
- Martin E., Tozer T. N., Sheiner L. B., Riegelman S. The clinical pharmacokinetics of phenytoin. J Pharmacokinet Biopharm. 1977 Dec;5(6):579–596. doi: 10.1007/BF01059685. [DOI] [PubMed] [Google Scholar]
- Metzler C. M., Tong D. D. Computational problems of compartment models with Michaelis-Menten-type elimination. J Pharm Sci. 1981 Jul;70(7):733–737. doi: 10.1002/jps.2600700706. [DOI] [PubMed] [Google Scholar]
- Mygind K. I., Dam M., Christiansen J. Phenytoin and phenobarbitone plasma clearance during pregnancy. Acta Neurol Scand. 1976 Aug;54(2):160–166. doi: 10.1111/j.1600-0404.1976.tb04789.x. [DOI] [PubMed] [Google Scholar]
- Nau H., Kuhnz W., Egger H. J., Rating D., Helge H. Anticonvulsants during pregnancy and lactation. Transplacental, maternal and neonatal pharmacokinetics. Clin Pharmacokinet. 1982 Nov-Dec;7(6):508–543. doi: 10.2165/00003088-198207060-00003. [DOI] [PubMed] [Google Scholar]
- Perucca E., Ruprah M., Richens A. Altered drug binding to serum proteins in pregnant women: therapeutic relevance. J R Soc Med. 1981 Jun;74(6):422–426. doi: 10.1177/014107688107400606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay R. E., Strauss R. G., Wilder B. J., Willmore L. J. Status epilepticus in pregnancy: effect of phenytoin malabsorption on seizure control. Neurology. 1978 Jan;28(1):85–89. doi: 10.1212/wnl.28.1.85. [DOI] [PubMed] [Google Scholar]
- Richens A. Drug estimation in the treatment of epilepsy. Proc R Soc Med. 1974 Dec;67(12 Pt 1):1227–1229. [PMC free article] [PubMed] [Google Scholar]
- Van Langenhove A., Costello C. E., Biller J. E., Biemann K., Browne T. R. A mass spectrometric method for the determination of stable isotope labeled phenytoin suitable for pulse dosing studies. Biomed Mass Spectrom. 1980 Nov;7(11-12):576–581. doi: 10.1002/bms.1200071126. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T. A nonlinear least squares program based on differential equations, MULTI (RUNGE), for microcomputers. J Pharmacobiodyn. 1983 Aug;6(8):595–606. doi: 10.1248/bpb1978.6.595. [DOI] [PubMed] [Google Scholar]


