Abstract
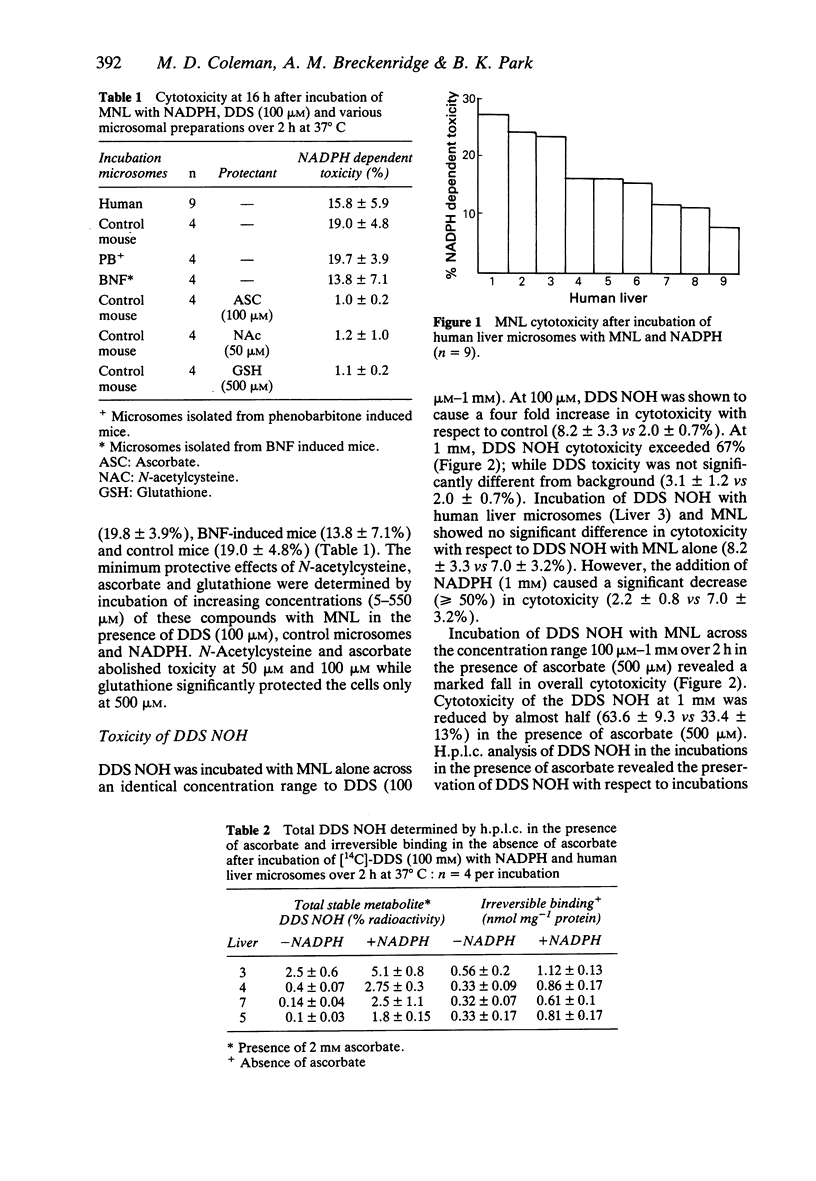
1. Using human mononuclear leucocytes as target cells, we have investigated the bioactivation of dapsone (DDS) to a cytotoxic metabolite in the presence of microsomes from nine human livers. Values for NADPH dependent toxicity ranged from 8.8-27% (15.8 +/- 5.9%) and were similar to those for microsomes from control mice, 16-24% (19.0 +/- 4.8%). 2. Microsomes prepared from mice induced with either phenobarbitone or beta-naphthoflavone did not produce significantly more NADPH dependent toxicity than microsomes prepared from control mice. 3. Cytotoxicity was abolished not only by ascorbic acid, but also by sub-physiological concentrations of N-acetylcysteine and glutathione. 4. DDS was metabolised in vitro to a hydroxylamine (metabolic conversion 3.1 +/- 1.5%), which was oxidised further to a cytotoxic metabolite which also became irreversibly bound to protein.
Full text
PDF


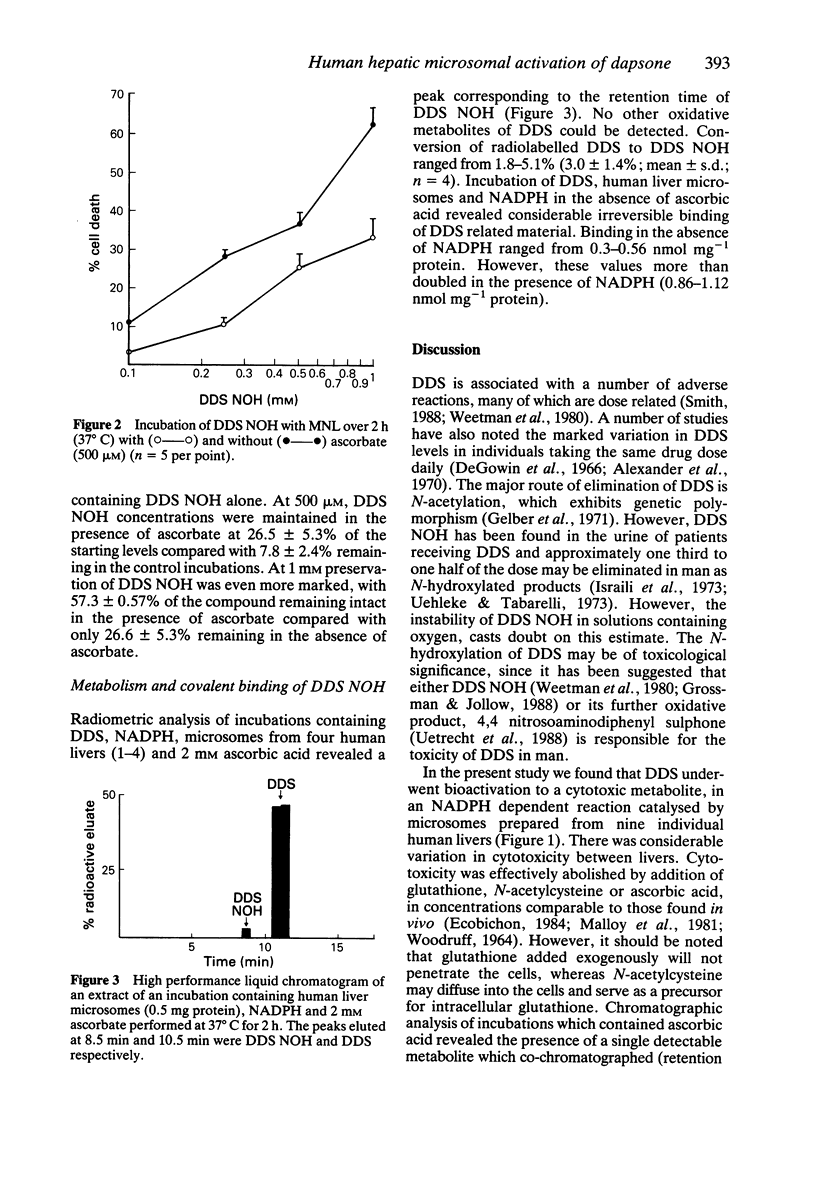


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. O., Young E., McFadyen T., Fraser N. G., Duguid W. P., Meredith E. M. Absorption and excretion of 35S dapsone in dermatitis herpetiformis. Br J Dermatol. 1970 Dec;83(6):620–631. doi: 10.1111/j.1365-2133.1970.tb15756.x. [DOI] [PubMed] [Google Scholar]
- BARNES J., BARNES E. J. Liver damage during treatment with diaminodiphenylsulfone. Lepr Rev. 1951 Jul-Oct;22(3-4):54–56. doi: 10.5935/0305-7518.19510008. [DOI] [PubMed] [Google Scholar]
- BEUTLER E., DURON O., KELLY B. M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888. [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Degowin R. L., Eppes R. B., Powell R. D., Carson P. E. The haemolytic effects of diaphenylsulfone (DDS) in normal subjects and in those with glucose-6-phosphate-dehydrogenase deficiency. Bull World Health Organ. 1966;35(2):165–179. [PMC free article] [PubMed] [Google Scholar]
- Ecobichon D. J. Glutathione depletion and resynthesis in laboratory animals. Drug Chem Toxicol. 1984;7(4):345–355. doi: 10.3109/01480548408998263. [DOI] [PubMed] [Google Scholar]
- Friman G., Nyström-Rosander C., Jonsell G., Björkman A., Lekås G., Svendsrup B. Agranulocytosis associated with malaria prophylaxis with Maloprim. Br Med J (Clin Res Ed) 1983 Apr 16;286(6373):1244–1245. doi: 10.1136/bmj.286.6373.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganer A., Knobel B., Fryd C. H., Rachmilewitz E. A. Dapsone-induced methemoglobinemia and hemolysis in the presence of familial hemoglobinopathy Hasharon and familial methemoglobin reductase deficiency. Isr J Med Sci. 1981 Aug;17(8):703–704. [PubMed] [Google Scholar]
- Gelber R., Peters J. H., Gordon G. R., Glazko A. J., Levy L. The polymorphic acetylation of dapsone in man. Clin Pharmacol Ther. 1971 Mar-Apr;12(2):225–238. doi: 10.1002/cpt1971122part1225. [DOI] [PubMed] [Google Scholar]
- Green S. T., Goldberg D. J., Leach J., Christie P. R., Kennedy D. H. AIDS-related Pneumocystis carinii pneumonia successfully treated with dapsone-trimethoprim. Br J Clin Pharmacol. 1988 Oct;26(4):487–488. doi: 10.1111/j.1365-2125.1988.tb03411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman S. J., Jollow D. J. Role of dapsone hydroxylamine in dapsone-induced hemolytic anemia. J Pharmacol Exp Ther. 1988 Jan;244(1):118–125. [PubMed] [Google Scholar]
- Israili Z. H., Cucinell S. A., Vaught J., Davis E., Lesser J. M., Dayton P. G. Studies of the metabolism of dapsone in man and experimental animals: formation of N-hydroxy metabolites. J Pharmacol Exp Ther. 1973 Oct;187(1):138–151. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang P. G., Jr Sulfones and sulfonamides in dermatology today. J Am Acad Dermatol. 1979 Dec;1(6):479–492. doi: 10.1016/s0190-9622(79)80088-2. [DOI] [PubMed] [Google Scholar]
- Malloy M. H., Rassin D. K., Gaull G. E. A method for measurement of free and bound plasma cyst(e)ine. Anal Biochem. 1981 May 15;113(2):407–415. doi: 10.1016/0003-2697(81)90095-6. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Purba H. S., Maggs J. L., Orme M. L., Back D. J., Park B. K. The metabolism of 17 alpha-ethinyloestradiol by human liver microsomes: formation of catechol and chemically reactive metabolites. Br J Clin Pharmacol. 1987 Apr;23(4):447–453. doi: 10.1111/j.1365-2125.1987.tb03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R. J., Maggs J. L., Lambert C., Kitteringham N. R., Park B. K. An in vitro study of the microsomal metabolism and cellular toxicity of phenytoin, sorbinil and mianserin. Br J Clin Pharmacol. 1988 Nov;26(5):577–588. doi: 10.1111/j.1365-2125.1988.tb05298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. L., Uetrecht J. P., Jones J. E. Cytotoxicity of oxidative metabolites of procainamide. J Pharmacol Exp Ther. 1987 Sep;242(3):833–841. [PubMed] [Google Scholar]
- Shear N. H., Spielberg S. P. In vitro evaluation of a toxic metabolite of sulfadiazine. Can J Physiol Pharmacol. 1985 Nov;63(11):1370–1372. doi: 10.1139/y85-225. [DOI] [PubMed] [Google Scholar]
- Smith W. C. Are hypersensitivity reactions to dapsone becoming more frequent? Lepr Rev. 1988 Mar;59(1):53–58. doi: 10.5935/0305-7518.19880009. [DOI] [PubMed] [Google Scholar]
- Spielberg S. P. Acetaminophen toxicity in human lymphocytes in vitro. J Pharmacol Exp Ther. 1980 May;213(2):395–398. [PubMed] [Google Scholar]
- Spielberg S. P., Gordon G. B., Blake D. A., Goldstein D. A., Herlong H. F. Predisposition to phenytoin hepatotoxicity assessed in vitro. N Engl J Med. 1981 Sep 24;305(13):722–727. doi: 10.1056/NEJM198109243051302. [DOI] [PubMed] [Google Scholar]
- Spielberg S. P. In vitro assessment of pharmacogenetic susceptibility to toxic drug metabolites in humans. Fed Proc. 1984 May 15;43(8):2308–2313. [PubMed] [Google Scholar]
- Swinson D. R., Zlosnick J., Jackson L. Double-blind trial of dapsone against placebo in the treatment of rheumatoid arthritis. Ann Rheum Dis. 1981 Jun;40(3):235–239. doi: 10.1136/ard.40.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehleke H., Tabarelli S. N-hydroxylation of 4,4'-diaminodiphenylsulphone (Dapsone) by liver microsomes, and in dogs and humans. Naunyn Schmiedebergs Arch Pharmacol. 1973;278(1):55–68. doi: 10.1007/BF00501863. [DOI] [PubMed] [Google Scholar]
- Uetrecht J. P. Reactivity and possible significance of hydroxylamine and nitroso metabolites of procainamide. J Pharmacol Exp Ther. 1985 Feb;232(2):420–425. [PubMed] [Google Scholar]
- Uetrecht J., Zahid N., Shear N. H., Biggar W. D. Metabolism of dapsone to a hydroxylamine by human neutrophils and mononuclear cells. J Pharmacol Exp Ther. 1988 Apr;245(1):274–279. [PubMed] [Google Scholar]
- Weetman R. M., Boxer L. A., Brown M. P., Mantich N. M., Baehner R. L. In vitro inhibition of granulopoiesis by 4-amino-4'-hydroxylaminodiphenyl sulfone. Br J Haematol. 1980 Jul;45(3):361–370. doi: 10.1111/j.1365-2141.1980.tb07156.x. [DOI] [PubMed] [Google Scholar]
- Woosley R. L., Drayer D. E., Reidenberg M. M., Nies A. S., Carr K., Oates J. A. Effect of acetylator phenotype on the rate at which procainamide induces antinuclear antibodies and the lupus syndrome. N Engl J Med. 1978 May 25;298(21):1157–1159. doi: 10.1056/NEJM197805252982101. [DOI] [PubMed] [Google Scholar]
- Zuidema J., Hilbers-Modderman E. S., Merkus F. W. Clinical pharmacokinetics of dapsone. Clin Pharmacokinet. 1986 Jul-Aug;11(4):299–315. doi: 10.2165/00003088-198611040-00003. [DOI] [PubMed] [Google Scholar]


