Abstract
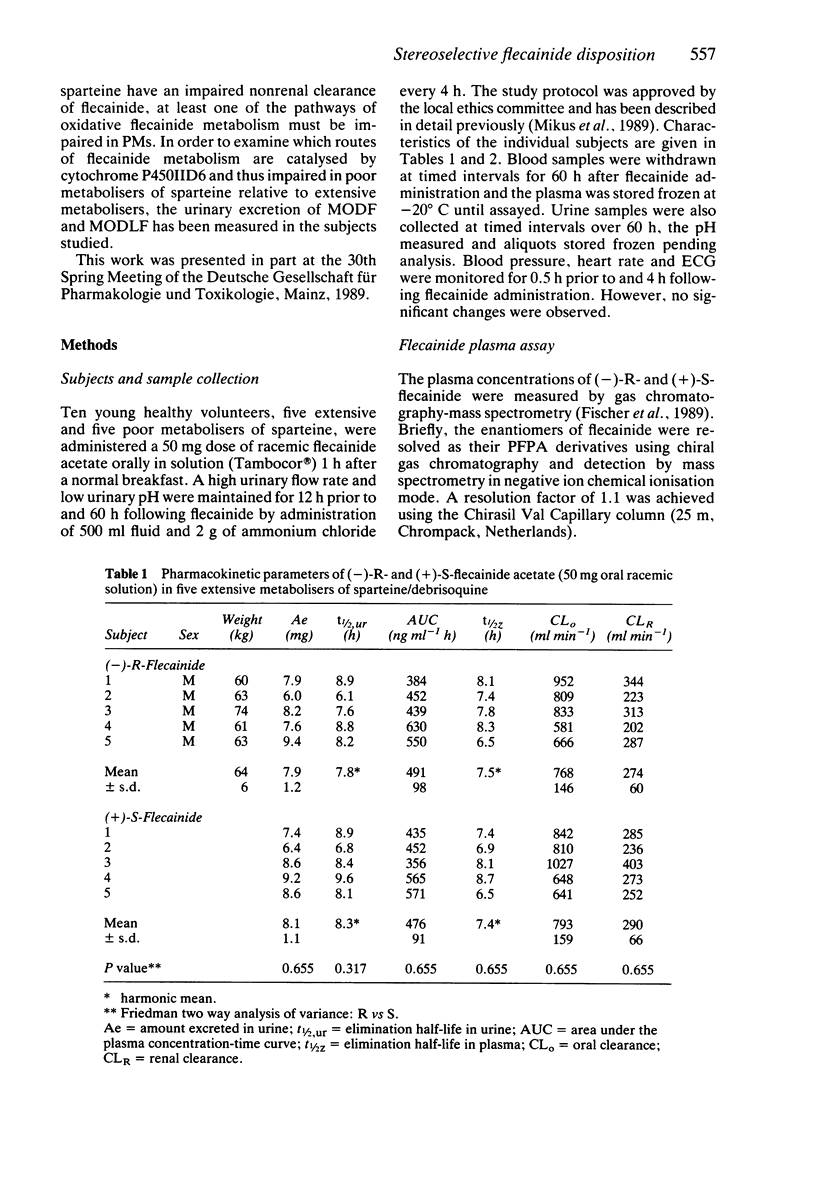
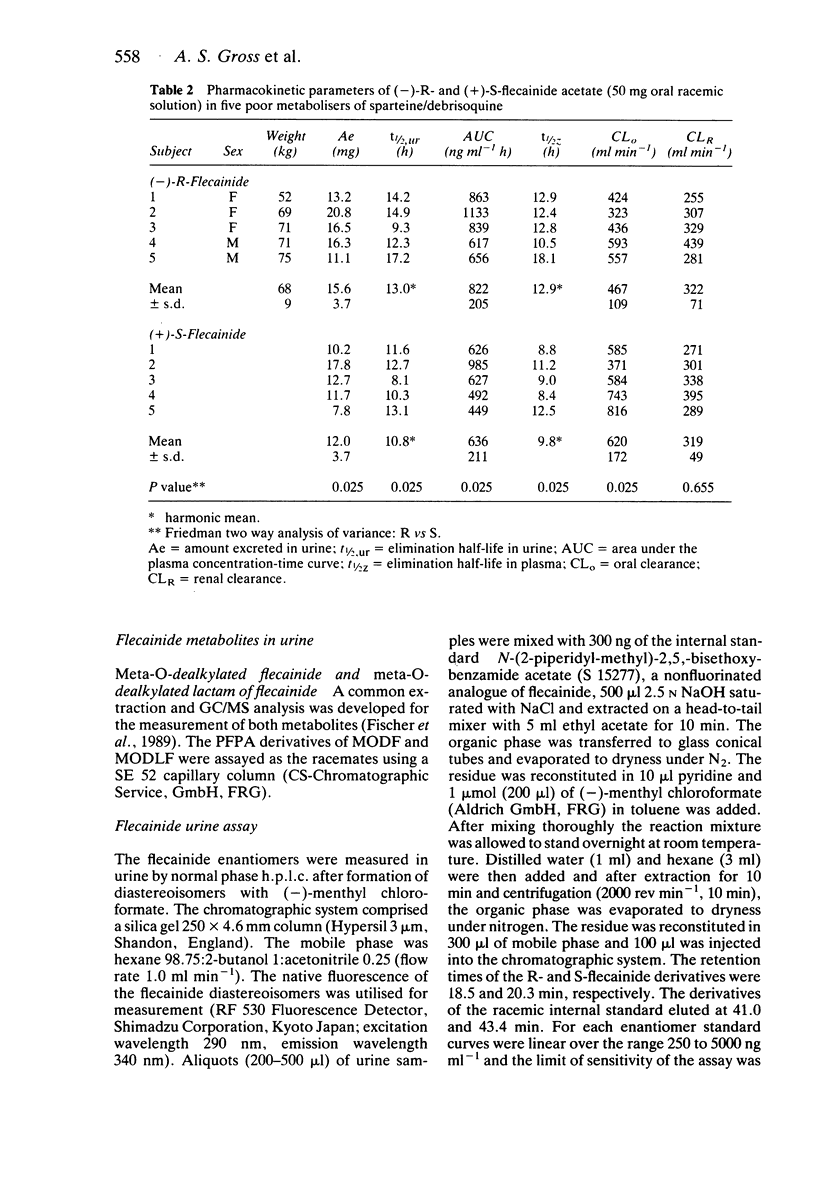
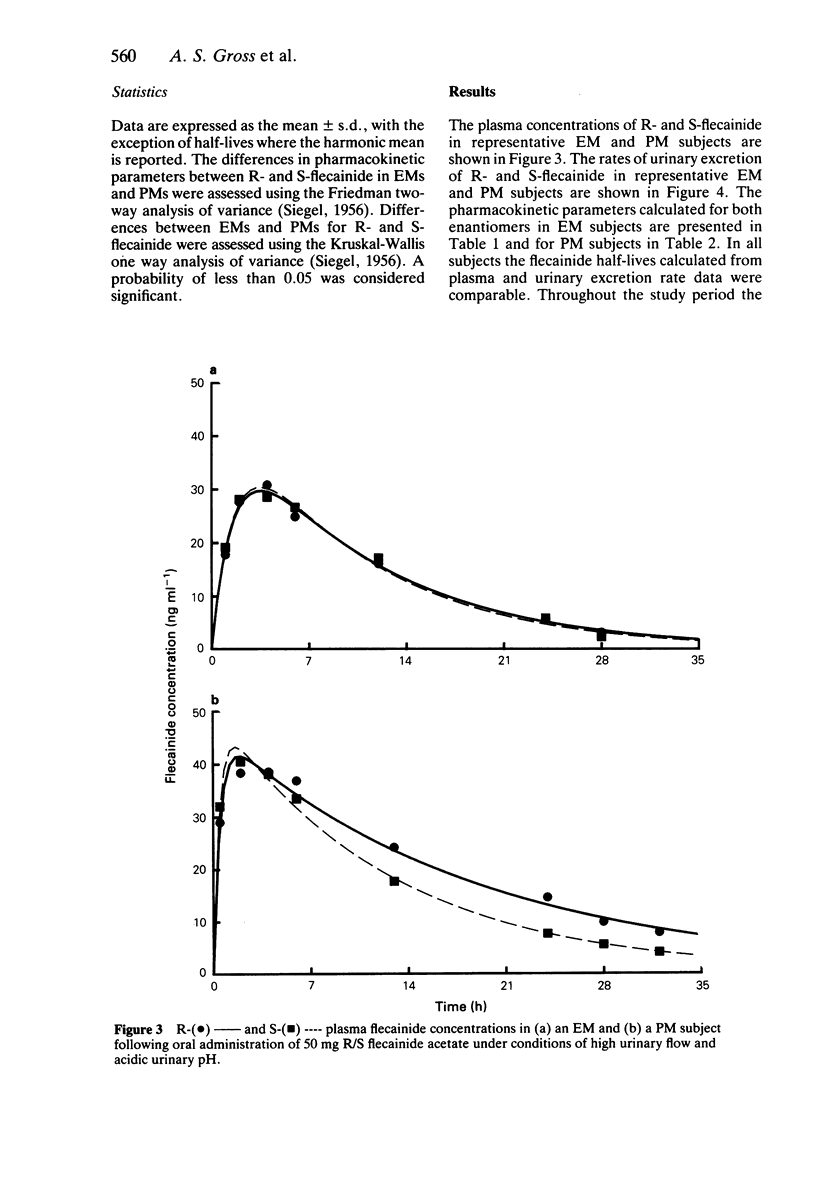
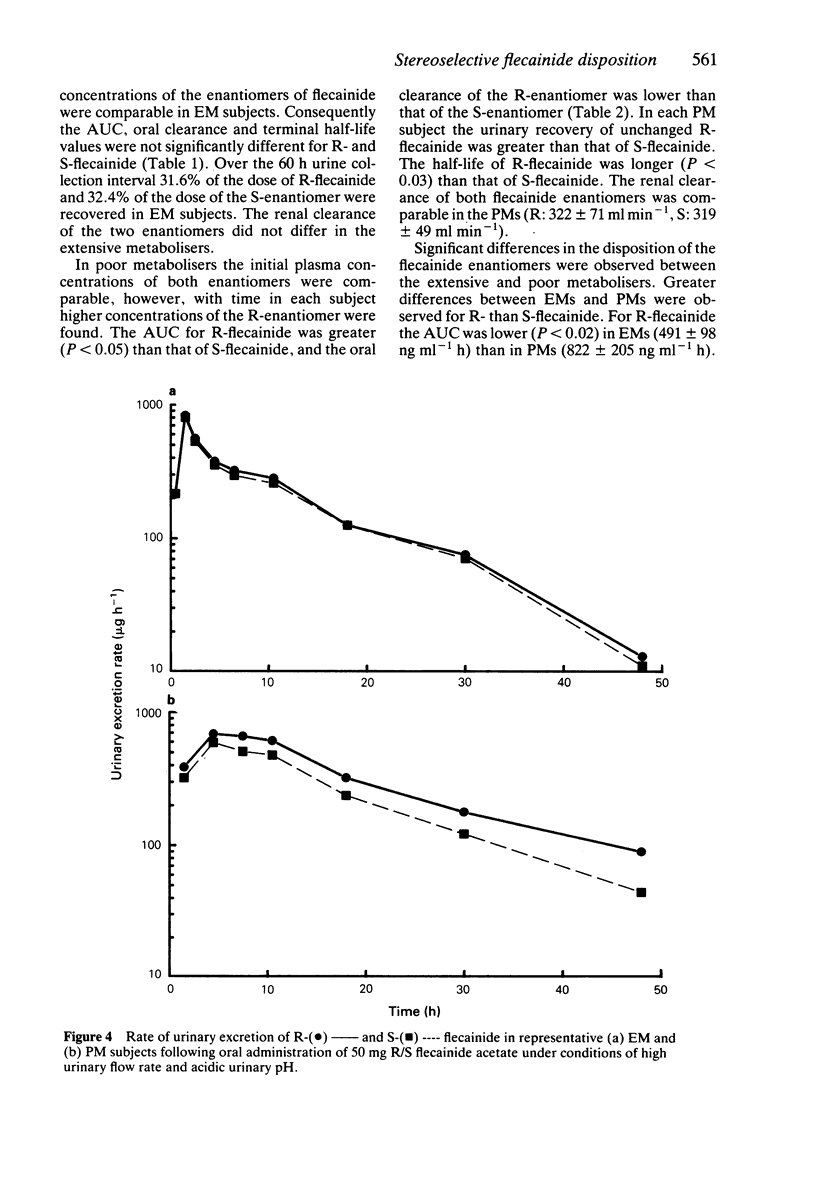
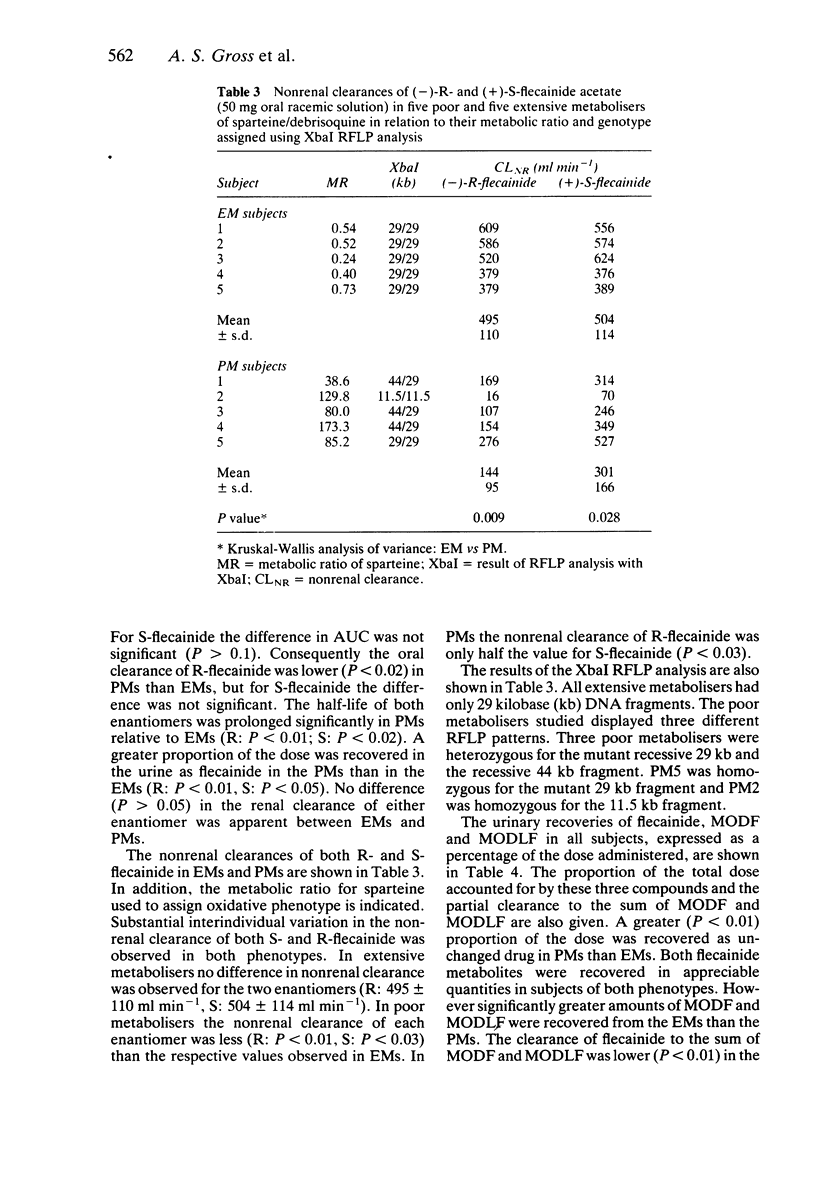
1. The disposition of the enantiomers of the antiarrhythmic drug flecainide has been studied in five extensive (EM) and five poor (PM) metabolisers of sparteine/debrisoquine after administration of 50 mg of racemic flecainide acetate under conditions of high urinary flow rate and acidic urinary pH. 2. In the EM subjects there were no significant differences in the oral clearance, half-life or urinary excretion of (+)-S- and (-)-R-flecainide. 3. In the PM subjects differences in the pharmacokinetics of S- and R-flecainide were observed. The oral clearance of R-flecainide (467 +/- 109 ml min-1) was less (P less than 0.03) than that of the S-enantiomer (620 +/- 172 ml min-1). The half-life of R-flecainide (12.9 h) was longer (P less than 0.03) than that of S-flecainide (9.8 h). The renal clearance of the two enantiomers was, however, comparable and similar to that observed in the EM subjects. The urinary recovery of R-flecainide (15.6 +/- 3.7 mg) was greater (P less than 0.03) than that of the S-enantiomer (12.0 +/- 3.7 mg). The enantioselective disposition observed in PMs is therefore due to greater impairment in the metabolism of R- than S-flecainide. 4. The urinary recoveries of two major metabolites of flecainide, meta-O-dealkylated flecainide (MODF) and the meta-O-dealkylated lactam of flecainide (MODLF) were lower (P less than 0.05) in PMs, 12.0% +/- 3.1% and 8.2% +/- 3.2% of the dose administered, respectively, than in EMs of 17.7% +/- 3.3% and 16.5% +/- 3.3%, respectively. 5. One PM subject had a greatly diminished flecainide metabolic capacity and a rare genotype, as assigned by Xbal RFLP analysis.
Full text
PDF
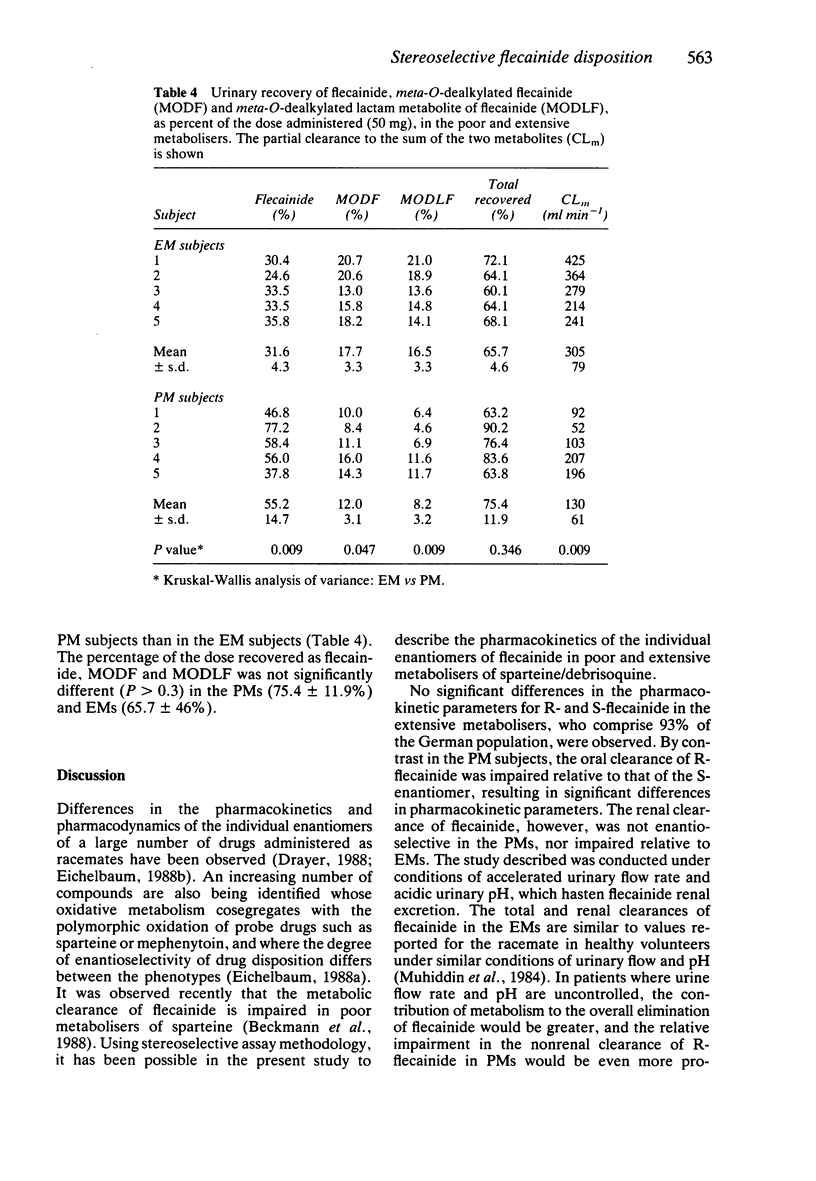



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banitt E. H., Schmid J. R., Newmark R. A. Resolution of flecainide acetate, N-(2-piperidylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)benzam ide acetate, and antiarrhythmic properties of the enantiomers. J Med Chem. 1986 Feb;29(2):299–302. doi: 10.1021/jm00152a021. [DOI] [PubMed] [Google Scholar]
- Beckmann J., Hertrampf R., Gundert-Remy U., Mikus G., Gross A. S., Eichelbaum M. Is there a genetic factor in flecainide toxicity? BMJ. 1988 Nov 19;297(6659):1316–1316. doi: 10.1136/bmj.297.6659.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Kollert J. R., Becker J. U. Pharmacokinetics of flecainide in patients with mild and moderate renal failure compared with patients with normal renal function. Eur J Clin Pharmacol. 1987;31(6):711–714. doi: 10.1007/BF00541300. [DOI] [PubMed] [Google Scholar]
- Cavalli A., Maggioni A. P., Marchi S., Volpi A., Latini R. Flecainide half-life prolongation in 2 patients with congestive heart failure and complex ventricular arrhythmias. Clin Pharmacokinet. 1988 Mar;14(3):187–188. doi: 10.2165/00003088-198814030-00007. [DOI] [PubMed] [Google Scholar]
- Chang S. F., Welscher T. M., Miller A. M., McQuinn R. L., Fox J. M. High-performance liquid chromatographic method for the quantitation of a meta-O-dealkylated metabolite of flecainide acetate, a new antiarrhythmic. J Chromatogr. 1985 Sep 13;343(1):119–127. doi: 10.1016/s0378-4347(00)84574-0. [DOI] [PubMed] [Google Scholar]
- Conard G. J., Ober R. E. Metabolism of flecainide. Am J Cardiol. 1984 Feb 27;53(5):41B–51B. doi: 10.1016/0002-9149(84)90501-0. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Bertilsson L., Küpfer A., Steiner E., Meese C. O. Enantioselectivity of 4-hydroxylation in extensive and poor metabolizers of debrisoquine. Br J Clin Pharmacol. 1988 Apr;25(4):505–508. doi: 10.1111/j.1365-2125.1988.tb03335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelbaum M. Pharmacokinetic and pharmacodynamic consequences of stereoselective drug metabolism in man. Biochem Pharmacol. 1988 Jan 1;37(1):93–96. doi: 10.1016/0006-2952(88)90758-7. [DOI] [PubMed] [Google Scholar]
- Forland S. C., Burgess E., Blair A. D., Cutler R. E., Kvam D. C., Weeks C. E., Fox J. M., Conard G. J. Oral flecainide pharmacokinetics in patients with impaired renal function. J Clin Pharmacol. 1988 Mar;28(3):259–267. doi: 10.1002/j.1552-4604.1988.tb03142.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Skoda R. C., Kimura S., Umeno M., Zanger U. M., Nebert D. W., Gelboin H. V., Hardwick J. P., Meyer U. A. Characterization of the common genetic defect in humans deficient in debrisoquine metabolism. Nature. 1988 Feb 4;331(6155):442–446. doi: 10.1038/331442a0. [DOI] [PubMed] [Google Scholar]
- Holmes B., Heel R. C. Flecainide. A preliminary review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1985 Jan;29(1):1–33. doi: 10.2165/00003495-198529010-00001. [DOI] [PubMed] [Google Scholar]
- Lennard M. S., Tucker G. T., Silas J. H., Freestone S., Ramsay L. E., Woods H. F. Differential stereoselective metabolism of metoprolol in extensive and poor debrisoquin metabolizers. Clin Pharmacol Ther. 1983 Dec;34(6):732–737. doi: 10.1038/clpt.1983.242. [DOI] [PubMed] [Google Scholar]
- McQuinn R. L., Pentikäinen P. J., Chang S. F., Conard G. J. Pharmacokinetics of flecainide in patients with cirrhosis of the liver. Clin Pharmacol Ther. 1988 Nov;44(5):566–572. doi: 10.1038/clpt.1988.195. [DOI] [PubMed] [Google Scholar]
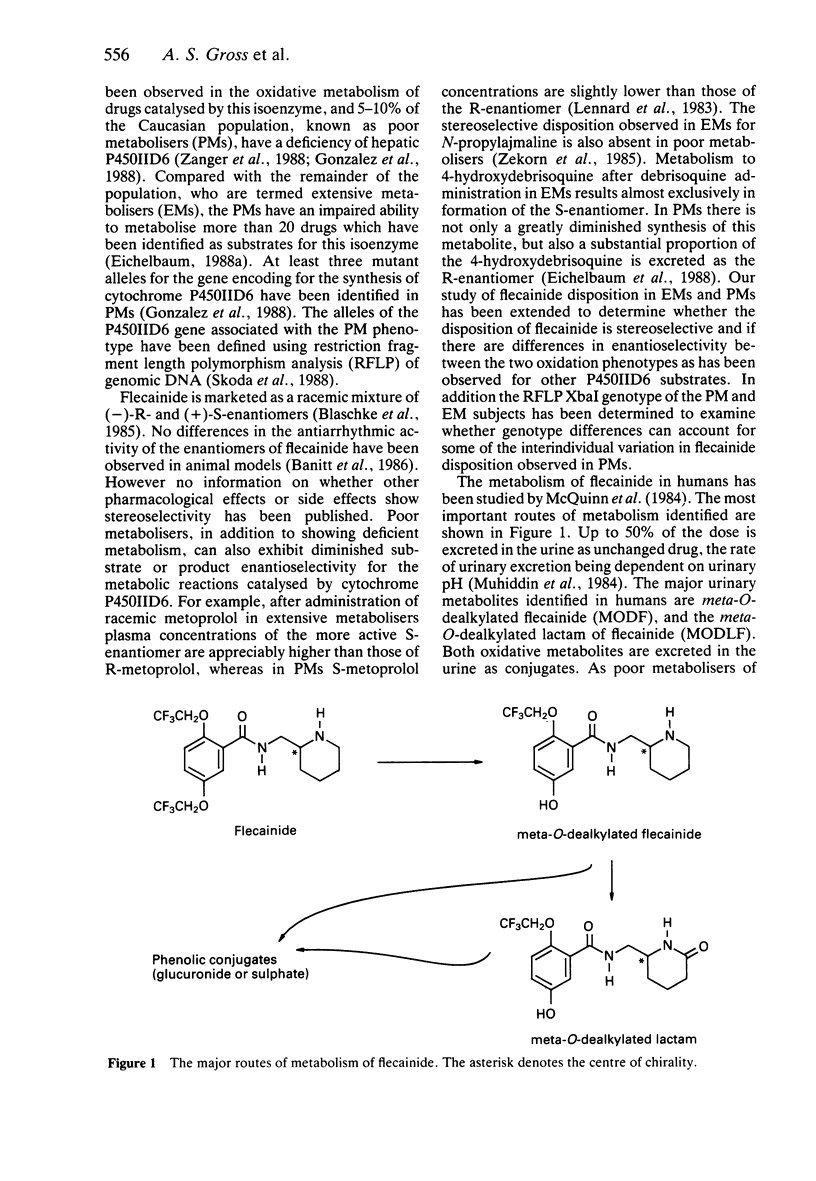
- McQuinn R. L., Quarfoth G. J., Johnson J. D., Banitt E. H., Pathre S. V., Chang S. F., Ober R. E., Conard G. J. Biotransformation and elimination of 14C-flecainide acetate in humans. Drug Metab Dispos. 1984 Jul-Aug;12(4):414–420. [PubMed] [Google Scholar]
- Mikus G., Gross A. S., Beckmann J., Hertrampf R., Gundert-Remy U., Eichelbaum M. The influence of the sparteine/debrisoquin phenotype on the disposition of flecainide. Clin Pharmacol Ther. 1989 May;45(5):562–567. doi: 10.1038/clpt.1989.73. [DOI] [PubMed] [Google Scholar]
- Muhiddin K. A., Johnston A., Turner P. The influence of urinary pH on flecainide excretion and its serum pharmacokinetics. Br J Clin Pharmacol. 1984 Apr;17(4):447–451. doi: 10.1111/j.1365-2125.1984.tb02370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch J., Neyses L., Köhler U., Lüderitz B. Erhöhte Flecainid-Plasmakonzentrationen bei Herzinsuffizienz. Dtsch Med Wochenschr. 1987 Oct 30;112(44):1698–1700. doi: 10.1055/s-2008-1068315. [DOI] [PubMed] [Google Scholar]
- Peck C. C., Barrett B. B. Nonlinear least-squares regression programs for microcomputers. J Pharmacokinet Biopharm. 1979 Oct;7(5):537–541. doi: 10.1007/BF01062394. [DOI] [PubMed] [Google Scholar]
- Roden D. M., Woosley R. L. Drug therapy. Flecainide. N Engl J Med. 1986 Jul 3;315(1):36–41. doi: 10.1056/NEJM198607033150106. [DOI] [PubMed] [Google Scholar]
- Salerno D. M., Granrud G., Sharkey P., Krejci J., Larson T., Erlien D., Berry D., Hodges M. Pharmacodynamics and side effects of flecainide acetate. Clin Pharmacol Ther. 1986 Jul;40(1):101–107. doi: 10.1038/clpt.1986.145. [DOI] [PubMed] [Google Scholar]
- Skoda R. C., Gonzalez F. J., Demierre A., Meyer U. A. Two mutant alleles of the human cytochrome P-450db1 gene (P450C2D1) associated with genetically deficient metabolism of debrisoquine and other drugs. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5240–5243. doi: 10.1073/pnas.85.14.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanger U. M., Vilbois F., Hardwick J. P., Meyer U. A. Absence of hepatic cytochrome P450bufI causes genetically deficient debrisoquine oxidation in man. Biochemistry. 1988 Jul 26;27(15):5447–5454. doi: 10.1021/bi00415a010. [DOI] [PubMed] [Google Scholar]
- Zekorn C., Achtert G., Hausleiter H. J., Moon C. H., Eichelbaum M. Pharmacokinetics of N-propylajmaline in relation to polymorphic sparteine oxidation. Klin Wochenschr. 1985 Nov 15;63(22):1180–1186. doi: 10.1007/BF01740595. [DOI] [PubMed] [Google Scholar]


