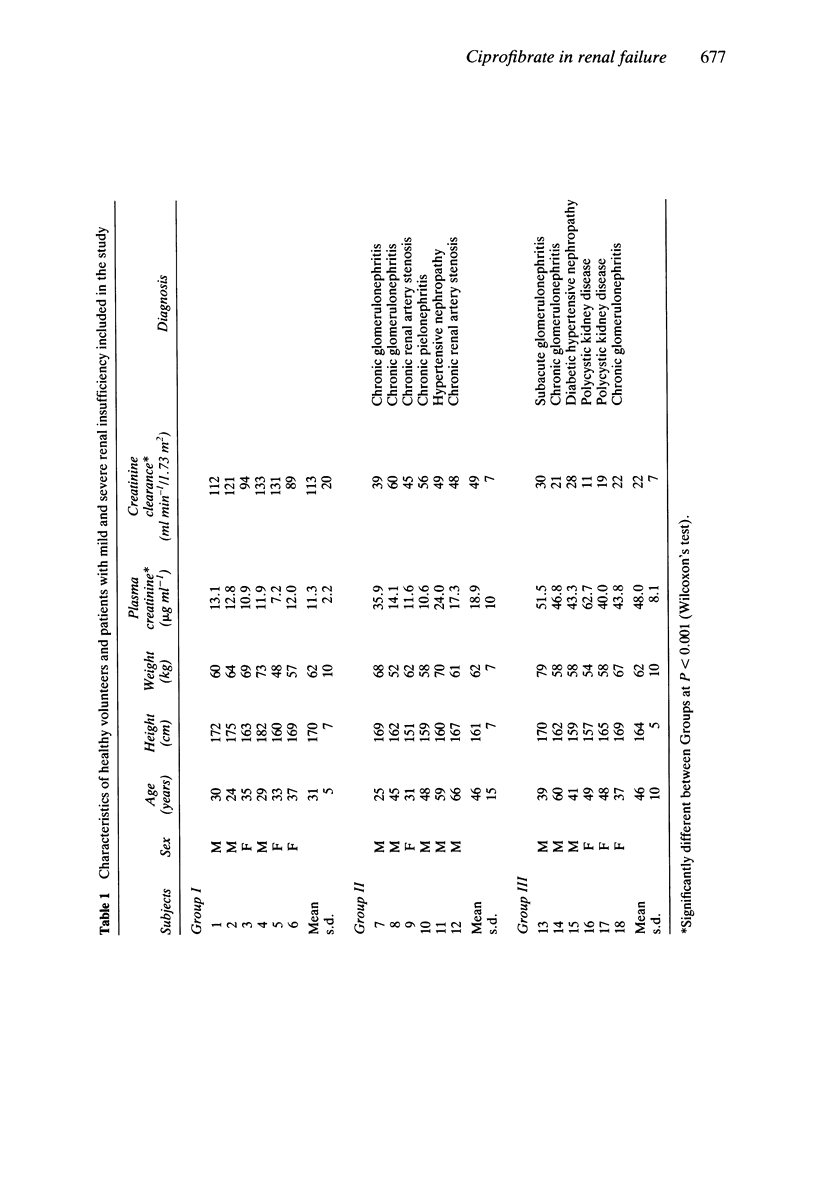
Abstract
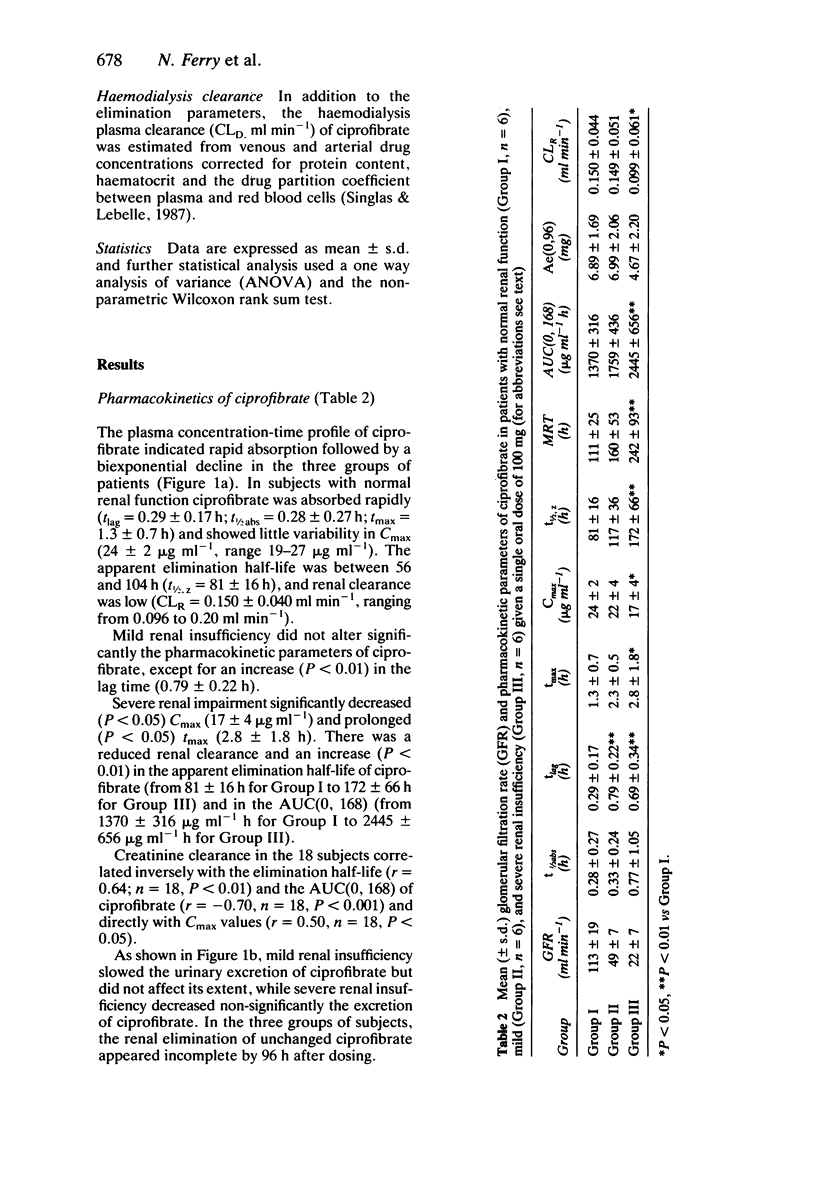
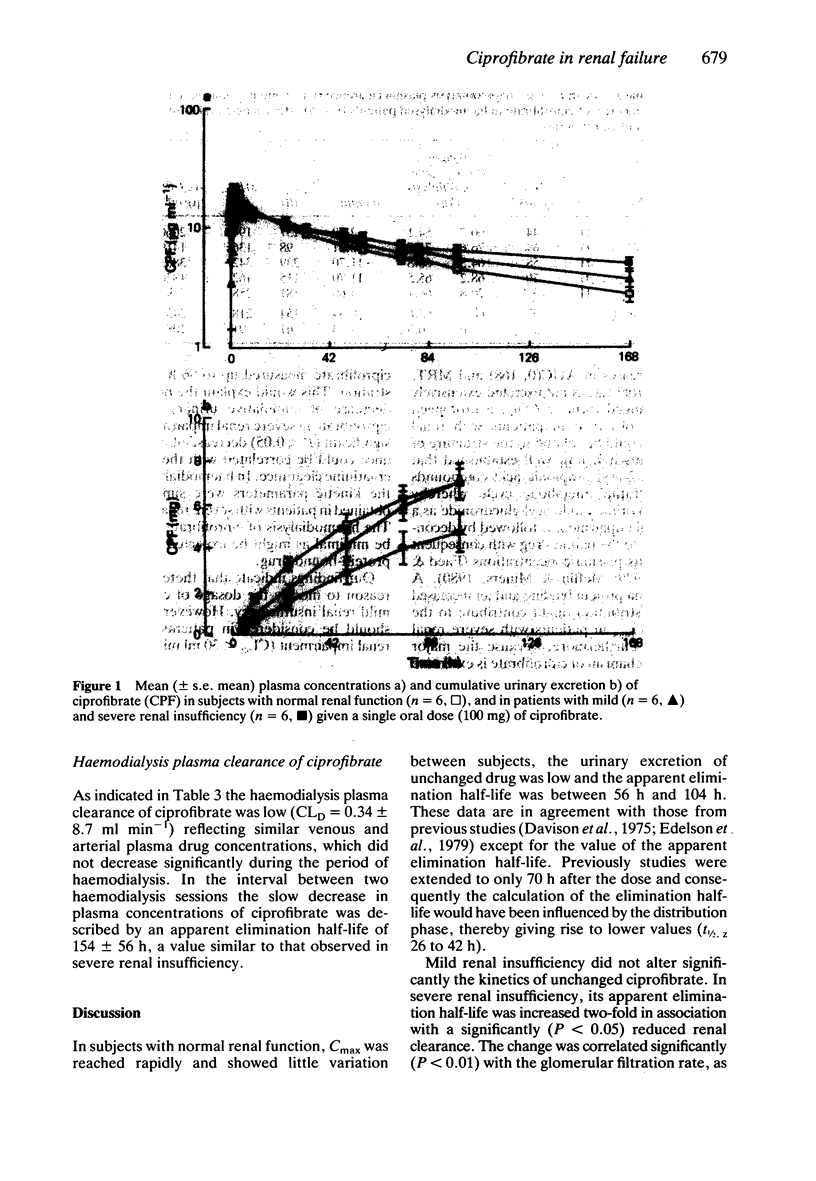
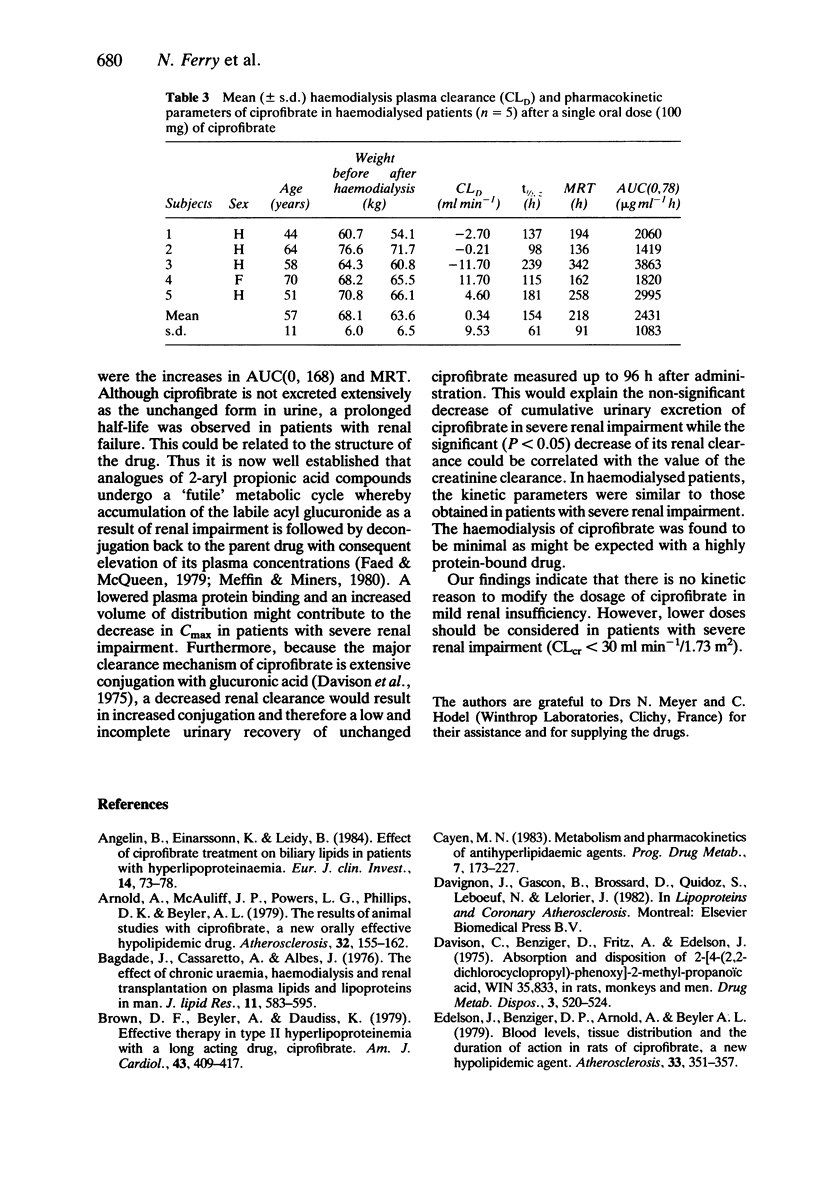
1. The kinetics of the hypolipidaemic drug, ciprofibrate, were studied after a single oral dose (100 mg) in subjects with normal renal function (n = 6), patients with mild (n = 6) and severe (n = 6) renal insufficiency as well as in haemodialysed patients (n = 5). 2. Under fasting conditions, ciprofibrate, was absorbed rapidly in subjects with normal renal function, and its apparent elimination half-life was approximately 81 h. Both renal clearance (0.15 ml min-1) and cumulative renal excretion (less than 7% of the administered dose) were low. 3. Mild renal insufficiency did not alter the pharmacokinetics of ciprofibrate, but severe renal impairment significantly reduced both its renal clearance and cumulative urinary excretion and increased the apparent elimination half-life. 4. A 5 h haemodialysis session did not lower the plasma concentrations of ciprofibrate. 5. It is concluded that, from a pharmacokinetic point of view, a reduction in the dosage of ciprofibrate should be considered in patients with a glomerular filtration rate below 30 ml min-1/1.73 m2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelin B., Einarsson K., Leijd B. Effect of ciprofibrate treatment on biliary lipids in patients with hyperlipoproteinaemia. Eur J Clin Invest. 1984 Feb;14(1):73–78. doi: 10.1111/j.1365-2362.1984.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Arnold A., McAuliff J. P., Powers L. G., Phillips D. K., Beyler A. L. The results of animal studies with ciprofibrate, a new orally effective hypolipidemic drug. Atherosclerosis. 1979 Feb;32(2):155–163. doi: 10.1016/0021-9150(79)90080-7. [DOI] [PubMed] [Google Scholar]
- Davison C., Benziger D., Fritz A., Edelson J. Absorption and disposition of 2-[4-(2,2-dichlorocyclopropyl)phenoxy]-2-methylpropanoic acid, WIN 35,833, in rats, monkeys, and men. Drug Metab Dispos. 1975 Nov-Dec;3(6):520–524. [PubMed] [Google Scholar]
- Edelson J., Benziger D. P., Arnold A., Beyler A. L. Blood levels, tissue distribution and the duration of action in rats of ciprofibrate, a new hypolipidemic agent. Atherosclerosis. 1979 Jul;33(3):351–357. doi: 10.1016/0021-9150(79)90186-2. [DOI] [PubMed] [Google Scholar]
- Elsom L. F., Hawkins D. R., Chasseaud L. F. Identification of a major metabolite of the new hypolipidaemic agent, isopropyl 2-(4'(p-chlorobenzoyl) phenoxy)-2-methylpropionate (procetofene) in humans by gas chromatography-mass spectrometry. J Chromatogr. 1976 Aug 4;123(2):463–467. doi: 10.1016/s0021-9673(00)82222-0. [DOI] [PubMed] [Google Scholar]
- Faed E. M., McQueen E. G. Plasma half-life of clofibric acid in renal failure. Br J Clin Pharmacol. 1979 Apr;7(4):407–410. doi: 10.1111/j.1365-2125.1979.tb00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G. B., Biddlecome C. E., Koblantz C., Edelson J. Determination of ciprofibrate in human plasma by high-performance liquid chromatography. J Chromatogr. 1982 Feb 12;227(2):534–539. doi: 10.1016/s0378-4347(00)80409-0. [DOI] [PubMed] [Google Scholar]
- Singlas E., Lebelle A. V. Extraction des médicaments par la dialyse. Therapie. 1987 Oct-Dec;42(6):529–540. [PubMed] [Google Scholar]
- Sirtori C. R., Franceschini G. Effects of fibrates on serum lipids and atherosclerosis. Pharmacol Ther. 1988;37(2):167–191. doi: 10.1016/0163-7258(88)90024-1. [DOI] [PubMed] [Google Scholar]
- Stamler J. Research related to risk factors. Circulation. 1979 Dec;60(7):1575–1587. doi: 10.1161/01.cir.60.7.1575. [DOI] [PubMed] [Google Scholar]
- Wülfert B., Majoie B., de Ceaurriz A. Antilipidemic drugs. Part 6: LF 178 in man. A preliminary note on a multicenter investigation bearing on 393 subjects with pure or mixed forms of hyperlipidemia. Arzneimittelforschung. 1976;26(5):906–909. [PubMed] [Google Scholar]


