Abstract
In the postnatal subventricular zone (SVZ) local cues or signaling molecules released from neuroblasts limit the proliferation of glial fibrillary acidic protein (GFAP)-expressing progenitors thought to be stem cells. However, signals between SVZ cells have not been identified. We show that depolarizations of neuroblasts induce nonsynaptic SNARE-independent GABAA receptor currents in GFAP-expressing cells whose time-course depends on GABA uptake in acute mouse slices. We found that GABAA receptors are tonically activated in GFAP-expressing cells consistent with the presence of spontaneous depolarizations in neuroblasts that are sufficient to induce GABA release. These data demonstrate the existence of nonsynaptic GABAergic signaling between neuroblasts and GFAP-expressing cells. Furthermore, we show that GABAA receptor activation in GFAP-expressing cells limits their progression through the cell cycle. Thus, as GFAP-expressing cells generate neuroblasts, GABA released from neuroblasts provides a feedback mechanism to control the proliferation of GFAP-expressing progenitors by activating GABAA receptors.
INTRODUCTION
The postnatal SVZ contains the largest pool of dividing neural progenitors in the adult brain. The SVZ consists of interconnected channels comprised of three major cell types1. The channel lumen is packed with neuroblasts. Many of these proliferate and migrate towards the olfactory bulb where they become interneurons2-5. GFAP-immunopositive progenitors, also called astrocytes1,6, line the channel walls and thus closely encapsulate neuroblasts. These GFAP-expressing cells constitute a pool of stem cells6-8. A small number of highly proliferative progenitors are present in clusters along the channels. Overall, the anterior SVZ is mostly composed of neuroblasts (∼60-70%) ensheathed by GFAP-expressing cells (∼20%), which are thus in a prime location to send and receive signals from neuroblasts. Furthermore, elimination of neuroblasts stimulates the proliferation of GFAP-expressing cells that regenerate the entire SVZ9, indicating that local cues or signaling molecules released from neuroblasts regulate the proliferation of GFAP-expressing cells. However, the signals between neuroblasts and GFAP-expressing cells have not been identified. Identifying these signals and their receptors is of considerable interest because they provide therapeutic targets for controlling the proliferation of GFAP-expressing progenitors and ultimately neurogenesis.
Among the possible signaling molecules, the neurotransmitter GABA is a prominent candidate for providing intercellular signals between neuroblasts and GFAP-expressing progenitors because SVZ progenitors synthesize and release GABA and GFAP-expressing cells express GABA transporters (GATs)10,11. GABA plays an important signaling role in developmental processes, such as embryonic cell proliferation and migration12-17 as well as regulating the migratory speed and production of neuroblasts in postnatal SVZ11,18. To support GABAergic signaling from neuroblasts to GFAP-expressing cells, the expression of GABA receptors in GFAP-expressing cells has yet to be demonstrated. Furthermore, it is unknown whether neuroblasts release GABA that activates GABA receptors on GFAP-expressing cells in a synaptic or nonsynaptic fashion, and whether this release depends on the classical SNARE complex. The SNARE complex, formed by the vesicle protein synaptobrevin/VAMP (R-SNARE) and by the plasmamembrane proteins SNAP25 and syntaxin (Q-SNARE), is the minimal machinery required for vesicle exocytosis. GABA release onto synaptically silent hippocampal neurons19 and from CA1 neurons20 is Q-SNARE-independent.
To determine whether neuroblasts communicate with GFAP-expressing cells via GABA signaling, we recorded from GFAP-expressing cells in the postnatal SVZ using transgenic mice expressing GFP driven by the human GFAP promoter (GFAPP-GFP). Our data demonstrate the existence of nonsynaptic, SNARE-independent GABAergic communication between neuroblasts and presumed stem cells. Furthermore, we found that GABAA receptor (GABAAR) inhibition increases the number of proliferative GFAP-expressing cells. Thus, GABA released from neuroblasts acts as a stop signal to limit GFAP-expressing cell proliferation, and this GABAergic communication between SVZ progenitors may contribute to maintaining a balance between amplification and mobilization of progenitors.
RESULTS
GFAP-expressing cells of the SVZ express functional GABAARs
Whole-cell patch-clamp recordings were performed in acute slices from postnatal transgenic GFAPP-GFP mice21. Fluorescent (GFP+) cells could be visualized before recording and then filled with lucifer yellow during recording to better examine their morphology. GFP+ cells recorded in the SVZ had cell bodies of irregular shape (∼10 μm-long) with one or two main processes (∼30-60 μm-long, Fig. 1a). Although striatal astrocytes adjacent to the SVZ are also GFP+, they were easily distinguished due to their more complex morphology (Supplemental Fig. 1) compared to GFP+ cells in the SVZ. Striatal astrocytes near the SVZ were characterized by several main processes confined to a 80-150 μm-diameter sphere or ellipse. GFP+ cells in the SVZ had a low mean input resistance (RIN, 50.9 ± 4.7 MΩ mean ± SEM, n = 48) and a hyperpolarized mean resting potential (VR, −80.7 ± 0.6 mV, n = 48) similar to those of astrocytes22 and radial glia23 but different from those of GFP-negative progenitors (mean RIN of ∼3 GΩ and zero-current VR of −28 mV), which are presumably neuroblasts11,24. We also performed immunostaining for nestin, a class VI intermediate filament protein, expressed in SVZ cells but not in astrocytes1,25. Lucifer yellow-filled GFP+ cells recovered after fixation of slices stained positive for nestin (n = 5/5, Fig. 1b), confirming that GFP+ cells with an immature morphology and recorded in the SVZ are the GFAP-expressing progenitors thought to be stem cells6-8.
Figure 1.

GFAP-expressing cells in the SVZ express functional GABAARs. (a) Photograph of a cell recorded at the edge of the SVZ (upper). The recorded cell was GFP+. Photograph of the lucifer yellow (LY)-fill of the recorded cell (below). (b) Photograph of a LY-filled GFP+ cell (green) immunostained for nestin (red), a marker of immature cells. (c) GABA-induced currents before, and during application of MFA (100 μM). (d) Currents in response to a 10-mV hyperpolarizing pulse before and during MFA application from the same cell as in (C). (e) GABA-induced currents before and during application of the GABAAR antagonist, gabazine (50 μM). (f) Current-voltage curve of GABA responses obtained by applying a ramp protocol (from –120 to 0 mV in 200 ms) near the peak of the current. GABA response reverses at −55 mV as expected for a Cl−-carried current in our recording conditions (ECl = −54 mV). GFP+ cells were recorded at a holding potential of –80 mV.
All GFAP-expressing cells examined responded to focal applications of GABA (100 μM). GABA induced inward currents that ranged from −20 to −300 pA in amplitude at a holding potential of −80 mV (n = 42, Fig. 1c). The variability in current amplitude is likely due to variability in the GABA concentration that reaches cells recorded at variable depths (50-150 μm) in the slice. GABA-induced currents slowly desensitized and were accompanied by an increase in noise, which was mostly visible in the presence of 100 μM meclofenamic acid (MFA), a gap junction blocker26. This increase in noise with MFA was accompanied by a decrease in GABA current amplitudes and a significant increase in RIN (to 305.4 ± 14.8 MΩ, n = 178, P < 0.001) without affecting the series (access) resistance (Fig. 1d). These effects were likely due to gap junction blockade by MFA26. Indeed, no dye coupling was observed in GFP+ cells recorded in the presence of MFA (Fig 1a, b) while GFP+ cells recorded without MFA displayed dye coupling to 4 or 8 surrounding GFP+ cells (data not shown). Although MFA affects responses at certain GABAARs27, MFA did not alter the amplitude of gabazine-sensitive currents in outside-out patches of GFAP-expressing cells (data not shown). MFA (100 μM) was then routinely bath applied after obtaining whole cell recordings of GFP+ cells to improve the space clamp of the recording. GABA responses in GFAP-expressing cells were reversibly blocked by the GABAAR antagonists, 20-50 μM gabazine (n = 5, Fig. 1e), 100 μM bicuculline (BIC) or 30-50 μM picrotoxin (PTX, n = 6 and 5, respectively, data not shown), and were mimicked by pressure application of a GABAAR agonist isoguvacine (10 μM, n = 3/3 and 100 μM, n = 3/3, data not shown). As expected for GABAAR-mediated (GABAA) currents, the mean reversal potential for GABA-induced currents (mean of −52.8 ± 6.8 mV, n = 7) was near the Nernst equilibrium potential for chloride (ECl= −54 mV with an intracellular [Cl-] of 18.5 mM, Fig. 1f). The mean EC50 of GABA was 15.1 μM (n = 5, Fig. 2a) obtained in cells pulled out and raised above the slice. Furthermore, single channel activity could be induced with 3 and 10 μM GABA in outside-out patches of GFAP-expressing progenitors (data not shown). The dose-dependent inhibition for PTX on 100 μM GABA responses gave an IC50 of 11.9 μM (n = 4, Fig. 2b).
Figure 2.
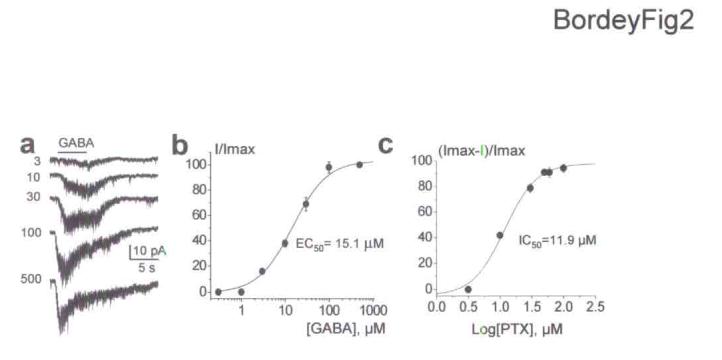
GABA and picrotoxin affinities for GABAARs in GFAP-expressing cells of the SVZ. (a) GABA responses induced by increasing GABA concentrations. (b-c) Mean dose-response curve for GABA (b) and picrotoxin (PTX, c) in GFAP-expressing cells gave an EC50 of 15.1 μM (n = 5) and an IC50 of 11.9 μM (n = 4), respectively. Four to six GABA concentrations were successively bath applied to each cell. The resulting averaged dose-response curve was fitted with a classical Hill equation. Errors bars represent s.e.m.
Stimulus-evoked nonsynaptic GABAAcurrents in GFAP cells
SVZ neuroblasts synthesize and contain GABA10,11. We thus set out to determine whether GABAARs could be activated by GABA released from SVZ progenitors following focal electrical stimulation in the SVZ. Single-pulse electrical stimulation in the SVZ failed to induce currents in GFAP-expressing cells (Fig. 3a). However, increasing the number of pulses (2-5 pulses at 50 Hz) induced slow, long-lasting inward currents of increasing amplitudes (Fig. 3a). These evoked inward currents were usually accompanied with an increase in noise and were reversibly reduced by BIC (100 μM, n = 3, Fig. 3b) and PTX (50 μM, n = 3, data not shown), suggesting that they were mediated by GABAAR activation in GFAP-expressing cells. In addition, evoked currents persisted in the presence of AMPA/kainate and NMDA receptor antagonists (20 μM DNQX and 50 μM D-APV, respectively, n = 5). Evoked GABAA currents ranged from −20 to −60 pA, suggesting that GABA release was small. Evoked currents displayed slower kinetics than those of synaptic currents recorded in other brain regions. Evoked currents developed slowly with a rise-time between 200 and 700 ms, and were long-lasting with a mean mono-exponential decay time constant (τ) of 1.16 ± 0.10 s (n = 24, Fig. 3c). Fast inward currents resembling synaptic currents were never observed following either a single or tetanic stimulation (Fig. 3c inset), or during bath application of 1 nM α-latrotoxin (Fig. 3d, n = 4). Although α-latrotoxin increased the frequency of spontaneous synaptic currents in striatal neurons (Fig. 3d, n = 6), no effect of α-latrotoxin in GFAP-expressing cells could be due to the lack of receptors necessary for the toxin internalization to the cytoplasm28.
Figure 3.
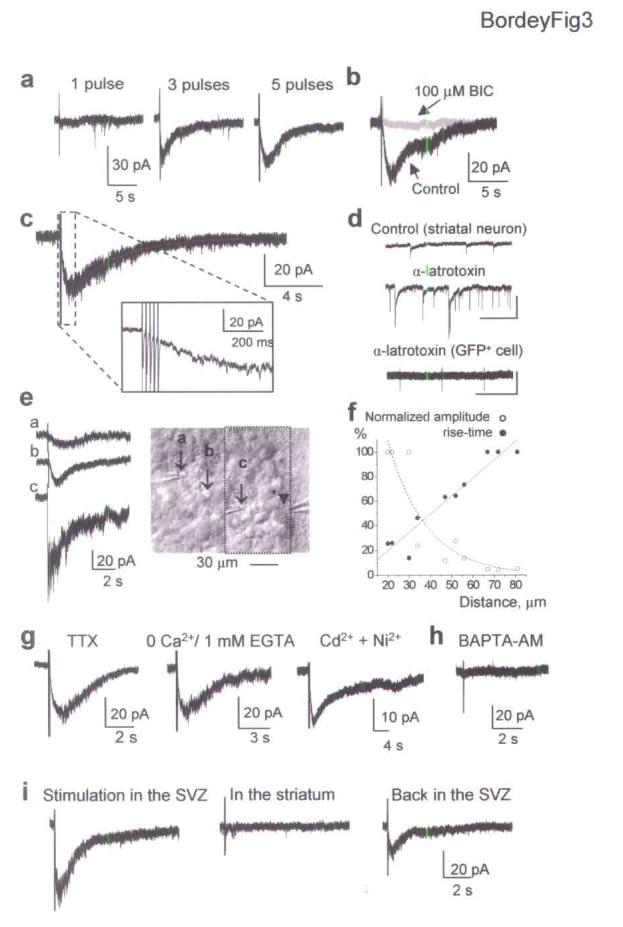
Electrical stimulation of SVZ progenitors evoked nonsynaptic GABAA currents in GFAP-expressing cells. (a) Repetitive focal electrical stimulation in the SVZ (1, 3 and 5 pulses of 200 μs, 50 Hz) induces inward currents in GFAP-expressing cells. (b) Currents evoked by a 5-pulse stimulus are reversibly blocked with bicuculline (BIC, 100 μM). (c) Evoked currents develop and decay slowly (Rise time: 680 ms and mono-exponential decay time constant: 1.7 s). Inset: trace at higher time scale. (d) Spontaneous synaptic events are observed in striatal neurons (top two traces) but not in GFAP-expressing cells (bottom trace) during 1 nM α-latrotoxin applications. Transient currents represent responses to 10-mV depolarizing pulses. Scale bars: 50 pA and 20 s (neuron), and 20 pA and 20 s (GFAP+ cell). (e) GABAA currents evoked by electrical stimulation at positions a, b and c shown (arrows) in the photograph. The arrowhead points to the recorded cell. (f) Plots of the normalized amplitudes and rise-times of evoked GABAA currents against the distance between the stimulating electrodes and recorded cells. (g) Evoked currents recorded with 1 μM TTX (left), in a 0-Ca2+ solution (30 min, middle), and in Cd2+ and Ni2+ for 30 min (each at 100 μM, right). (h) Failure to evoke currents recorded in slices incubated (30 min prior recording) in a Ca2+-free solution containing BAPTA-AM. (i) Focal electrical stimulation in the SVZ induces inward currents in GFAP-expressing cells recorded midway in the SVZ while no current was induced with an identical stimulation in the striatum.
To further ensure that GABA released by SVZ progenitors diffuses and activates receptors in GFAP-expressing cells in a nonsynaptic manner, we tested whether the amplitude and kinetics of evoked currents changed with the distance between the stimulating electrodes and recorded cells. The rise-time of evoked GABAA currents decreased and amplitude increased when the stimulating electrode was moved closer to the recorded cell (Fig. 3e,f). Stimulating electrodes were usually placed 40 to 60 μm from the recorded cell (between positions b and c in Fig. 3e) close to the lateral ventricle and GFP+ cells were recorded midway in the SVZ except for experiments displayed in Figure 3e. Variability in the distance between the stimulating electrode and recorded cell, and the presence of GATs (see Fig. 4) likely contribute to the variability in the evoked current amplitude and kinetics. We next examined whether evoked currents were independent of action potentials and extracellular or intracellular Ca2+. Evoked GABAA currents in GFAP-expressing cells persisted in the presence of the voltage-dependent sodium channel blocker, tetrodotoxin (TTX, 1 μM, n = 4), in a Ca2+-free extracellular solution applied for 30 min (0 mM Ca2+ plus 1 or 2 mM EGTA, n = 4), or in the presence of the voltage-gated Ca2+ channel blockers, Ni2+ and Cd2+ (each at 100 μM, n = 4, Fig. 3g). These data suggested a nonsynaptic release of GABA. TTX, Ni2+ and Cd2+ or a Ca2+-free solution abolished or significantly reduced GABAA synaptic currents in Purkinje neurons induced by one pulse stimulation in the molecular layer (data not shown). When slices were incubated for 30 min in 30 μM BAPTA-AM in a Ca2+-free solution to buffer intracellular Ca2+, no evoked currents could be induced in GFAP-expressing progenitors (n = 9 cells, 72 stimulations, 5 slices, Fig. 3h). Nevertheless, GABA responses could still be induced in SVZ progenitors by exogenous GABA applications (n = 4, data not shown). These data suggest that increases in intracellular Ca2+ levels are necessary for inducing GABA release. Consistent with a nonsynaptic release, we found no immunostaining for synapsin I near GFAP-immunopositive cells in the SVZ (Supplemental Fig. 2). Synapsin I is a specific marker of synapses29. Because the striatum contains GABAergic terminals, we next examined whether tetanic stimulation in the striatum adjacent to the SVZ induced GABA responses in GFAP-expressing cells. While tetanic stimulation in the SVZ induced inward currents in GFAP-expressing cells recorded midway in the SVZ, the same stimulation applied in the striatum within the same distance from the recorded cells failed to induce such currents (n = 4, Fig. 3i). Although GABA is released from synaptic terminals in the striatum, dilution by diffusion and uptake into both astrocytes and GFAP-expressing cells likely prevent significant levels of synaptically released GABA to reach recorded cells in the SVZ. These data indicate that GABA or a GABAAR agonist is nonsynaptically released from SVZ progenitors and activates GABAARs in GFAP-expressing cells.
Figure 4.
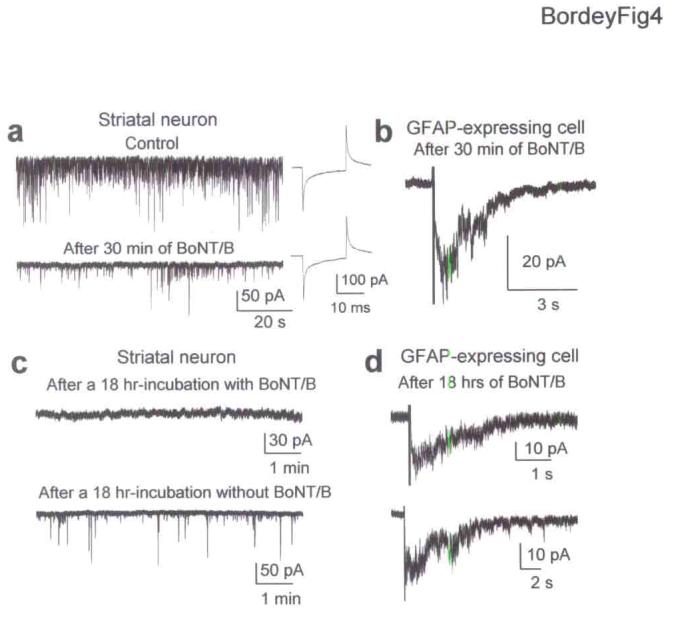
GABA release from SVZ progenitors is SNARE-independent. (a) Spontaneous synaptic currents in a striatal neuron under control conditions and after 30 min application of 200 ng/ml BoNT/B. BoNT/B did not affect series resistance or cell capacitance measured by applying a 10 mV-hyperpolarizing pulse before and after BoNT/B application. (b) Evoked GABAA currents in GFAP-expressing cells after a 30 min-application of BoNT/B. (c) Records of synaptic activity in striatal neurons in slices incubated for 18 hrs in a solution with or without 100 ng/ml BoNT/B. (d) Evoked GABAA currents in GFAP-expressing cells in slices treated with BoNT/B for 18 hrs.
SNARE-independent GABA release from SVZ progenitors
Because evoked GABA currents were dependent on intracellular Ca2+, we examined whether GABA release was abolished after inhibition of the SNARE complex. We incubated slices with botulinum neurotoxin A (BoTN/A) or B (BoTN/B) to block the Q- or R-SNARE complex, respectively. Bath applications of BoNT/A or B (each at 200 ng/ml) for 30 min significantly (P < 0.001) reduced the frequency of spontaneous gabazine (20 μM)-sensitive synaptic currents by 80% in striatal neurons without affecting cell capacitance and RIN (n = 4, Fig. 4a). Synaptic activity was studied in the presence of AMPA/kainate and NMDA receptor antagonists (20 μM DNQX and 50 μM D-APV). However, similar treatment with BoNT/A or B for 30 min did not affect evoked GABAA currents in GFAP-expressing cells (n = 4 for each toxin, Fig. 4b). Treated and control slices were then incubated for 18 hrs in a solution containing 100 ng/ml of BoNT/A or BoNT/B (without MFA) and no toxin, respectively. Spontaneous GABAergic synaptic currents were absent in neurons recorded in treated slices but were present in neurons from control slices (Fig. 4c for BoNT/B. data not shown for BoNT/A). Furthermore,α-latrotoxin (1 nM) did not induce spontaneous synaptic currents in neurons from treated slices (n = 5, data not shown). Evoked slow GABAA currents in GFAP-expressing cells persisted in slices treated with either toxin (n = 5 and 6 with BoNT/A and B, respectively, Fig. 4d for BoNT/B). These data suggest that GABA release from SVZ progenitors is SNARE-independent.
GABA uptake controls GABAAR activation in GFAP cells
Four subtypes of GATs, called mouse GAT1 to GAT4 have been reported30. GAT2 is less widespread in the brain and has the lowest affinity for GABA31. Bath application of non-transportable antagonists of the three major GATs, 50 μM NO-711 for GAT1 and 50 μM SNAP5114 for GAT3/4, significantly enhanced the amplitude and prolonged the decay of PTX- and BIC-sensitive inward currents evoked by tetanic stimulation (n = 5, P < 0.05, Fig. 5a,b). Furthermore, NO-711 and SNAP5114 induced inward shifts of the holding current of about −5 to −20 pA (n = 6/10, Fig. 5c). These results suggest that GATs decrease ambient GABA levels and limit GABAAR activation in GFAP-expressing cells. To determine whether both GAT1 and GAT3/4 contributed to the clearance of GABA, we tested the effects of each blocker on GABA responses induced by pressure applications of GABA (100 μM, 100 ms, Fig. 5e). SNAP5114 significantly (P < 0.05) increased the amplitude of GABA-induced responses by 61 ± 39% in 3/5 cells while NO711 had no effect on the amplitude (n = 5, Fig. 5e). Both SNAP5114 and NO-711 significantly increased τ of GABA responses by 91 ± 26% (n = 7) and 33 ± 15% (n = 5), respectively (P < 0.05, Fig. 5f,g). These data suggest that GAT3/4 transporters are more efficient than GAT1 transporter at regulating ambient and nonsynaptically released GABA levels in the SVZ.
Figure 5.
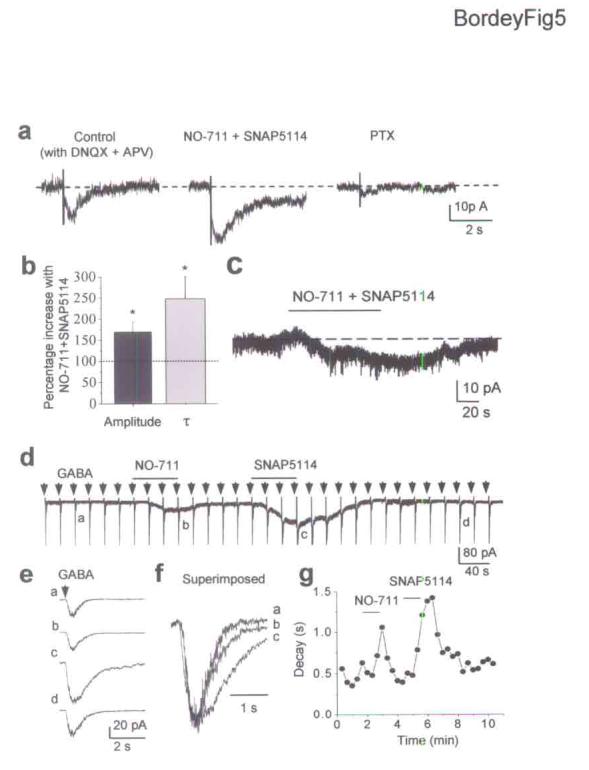
GABA uptake controls GABAAR activation in GFAP-expressing cells of the SVZ. (a) Bath application of GABA transporter antagonists, 50 μM NO-711 (for GAT1) and 50 μM SNAP5114 (for GAT3/4) enhances the amplitude and prolongs the decay time of PTX-sensitive currents evoked by tetanic stimulation in the SVZ. (b) Percentage increases of the evoked GABAA current amplitude and decay time constant in the presence of NO-711 and SNAP5114. Errors bars represent s.e.m. (c) NO-711 and SNAP5114 (50 μM each) reversibly induce an inward shift of the holding current in an GFAP-expressing cell. (d) Inward currents evoked by pressure applications of GABA (100 μM, 100 ms, every 20 s) before and during bath application of NO-711 (50 μM) and SNAP5114 (50 μM). Note that GABA responses were preceded by short 10 mV-depolarizations (transients on the records) to monitor the series and input resistance. In addition, NO-711 and SNAP5114 induced inward currents, suggesting that GABA transporter inhibition induced an increase in ambient GABA levels. (e) Average trace of three successive GABA responses at a higher time scale under control (a), in the presence of NO-711 (b) or SNAP5114 (c) and after washout (d). (f) Superimposed GABA responses (averaged) under control (a) and with NO-711 (b) or SNAP5114 (c). (g) Mono-exponential decay time constant of GABA response against the recording time.
Depolarization-induced GABAA currents in GFAP cells
We examined whether a subtle and near-physiological depolarizations of SVZ progenitors induces GABAAR activation in GFAP-expressing cells. Bath applications of 7.5 and 12.5 mM K+ or focal (pressure) applications of 12.5 mM K+ depolarized neuroblasts by 10 and 15 mV, respectively (n = 9, without MFA in the bath, data not shown). Bath and pressure applications of K+, which depolarizes every progenitor or a local group of SVZ progenitors, respectively, induced inward currents in GFAP-expressing cells that were reversibly reduced by BIC (n = 8, Fig. 6a) or PTX (n = 6, Fig. 6b). Neither PTX nor BIC affected the series (access) resistance or capacitance calculated in response to a 10 mV-depolarizing pulse (Fig. 6b for PTX). As a control for high K+ application, application of a solution into which 5 mM sucrose was added induced no currents in GFAP-expressing cells (n = 3). As expected for GABAA currents, the mean reversal potentials for high K+-induced GABAA currents were −58.7 ± 2.3 mV and +1.9 ± 1.4 mV (n = 6, data not shown) when using a low (ECl = −54 mV) and high internal Cl− solution (ECl = 1.3 mV), respectively. High K+-induced GABAA currents persisted in the presence of TTX (data not shown) or in a Ca2+-free solution (n = 4, Fig. 6a). Furthermore, no fast, transient currents resembling synaptic currents were observed during high K+ applications. PTX-sensitive currents induced by 12.5 mM K+ (bath) had significantly larger amplitudes (−34.0 ± 5.8 pA) than those induced by 7.5 mM K+ (−22.0 ± 4.0 pA, n = 6, P < 0.05). Focal (pressure) application of 12.5 mM K+ induced GABAA currents of −10 to −20 pA (n = 5). High K+ applications also induced BIC- and PTX-insensitive currents (Fig. 6a,b). Because GFAP-expressing cells have a high resting K+ conductance like astrocytes (data not shown), BIC- and PTX-insensitive inward currents induced by K+ likely represent a change in the resting K+ current due to a shift in the Nernst equilibrium potential for K+.
Figure 6.
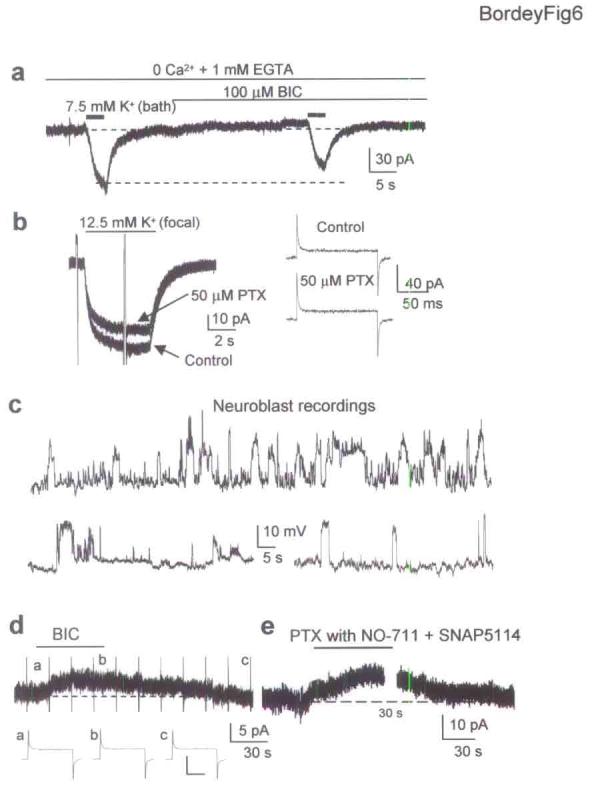
Near-physiological depolarization of SVZ progenitors induces activation of GABAARs in GFAP-expressing cells that are also tonically activated. (a) Bath application of 7.5 mM K+ induces BIC-sensitive inward currents in an GFAP-expressing cell recorded in a Ca2+-free solution. (b) Focal pressure application of 12.5 mM K+ to a cluster of putative neuroblasts induced PTX-sensitive currents in GFAP-expressing cells. High K+ applications are not accompanied by changes in series (access) resistance or capacitance calculated in response to a 10 mV-depolarizing pulse applied before and during PTX application. (c) Spontaneous depolarizations of 15-20 mV in neuroblasts at −60 mV. (d) 100 μM BIC induces a 5 pA-outward shift of the holding current in an GFAP-expressing cell without affecting series resistance or cell capacitance measured by applying a 10 mV-depolarizing pulse before, during and after BIC application. The extracellular solution contained 2.5 mM K+. Scale bar: 100 pA, 20 ms. (e) 50 μM PTX induces a 20 pA-outward shift of the holding current in an GFAP-expressing cell in the presence of NO-711 and SNAP5114 (50 μM each).
We next examined whether 10 to 15 mV-depolarizations spontaneously occurred in neuroblasts. Neuroblasts were recorded in current clamp from −60 mV, which is near their estimated resting potential10, and without MFA. Nevertheless, MFA had no effect on the resting potential, RIN, and the spontaneous depolarizations observed in neuroblasts (data not shown). 71% of the neuroblasts displayed spontaneous depolarizations of 15 to 20 mV (Fig. 6c), which occurred at a frequency of 0.3 to 12/min and were insensitive to GABAAR blockers. These large spontaneous depolarizations were observed during the whole length (45-75 min) of stable whole-cell and perforated recordings, suggesting that they were not due to cell damage. Spontaneous single channel currents of −5 pA-amplitude that correspond to a 15 to 20 mV depolarization due to the large RIN of neuroblasts (3-4 GΩ) were also observed in voltage clamped neuroblasts. 40% of the neuroblasts also displayed small gabazine-sensitive depolarizations of ∼5-8 mV (data not shown), consistent with tonic GABAAR activation in neuroblasts11,18. These results show that K+-induced depolarizations of SVZ progenitors similar in amplitude to the spontaneous depolarizations found in SVZ neuroblasts can induce nonsynaptic GABAAR activation in surrounding GFAP-expressing cells.
Neuroblasts mediate tonic GABAA current in GFAP cells
The presence of spontaneous depolarizations in neuroblasts suggests that GABAARs are tonically activated in some GFAP-expressing cells due to spontaneous GABA release. Bath application of BIC (100 μM, Fig. 6d) or PTX (50 μM, Fig, 6e) induced reversible +5 to +30 pA outward shifts of the holding currents when recorded in a 2.5 or 5 mM K+ extracellular solution with and without GABA transporter inhibitors, 50 μM NO-711 and 50 μM SNAP5114 (n = 12/17 and n = 7/12, respectively). This outward shift was not accompanied by a change in series resistance or cell capacitance (Fig. 6d). These results indicate that there is sufficient ambient GABA in the SVZ to tonically activate GABAARs in GFAP-expressing cells.
Although electrical and high K+ stimulations indiscriminately depolarize all SVZ cells, ∼70% of the SVZ cells that stain positive for GABA are neuroblasts11, suggesting that neuroblasts are the major source of GABA in the SVZ. Furthermore, GFP+ cells did not stain positive for GABA (Fig. 7a). To conclusively show that neuroblasts release GABA spontaneously and upon depolarization, we used outside-out patches of striatal neurons or GFAP-expressing cells held at −80 mV to monitor GABAA single channels (without MFA) and detect GABA release from simultaneously recorded neuroblasts (Fig. 7b-e). Neuroblasts were recorded in the cell-attached mode to keep the intracellular milieu intact. Cell-attached patches were held at a pipette potential (Vpip) of 0 mV (patch potential Vpatch = VR, using Vpatch = VR−Vpip). Experiments were performed with AMPA/kainate and NMDA receptor blockers (DNQX and D-APV). Because gabazine (20 μM)-sensitive single channel activity was detected when outside-out patches were ∼5 μm above the slices, outside-out patches were raised at ∼50 μm above the slice where no single channel activity was observed (Fig. 7c). The cell-attached neuroblasts were at first positioned at 50 μm from the patches and from the slice. A total of 21 paired recordings (4 neuron- and 17 GFAP-expressing cell-neuroblast pairs) were obtained. 52% of the outside-out patches (7 from GFAP-expressing cells and 4 from striatal neurons) displayed single channel activity when placed < 5 μm from the recorded neuroblast (Fig. 7b,c,d). This activity was abolished by 20 μM gabazine in outside-out patches from neurons (n = 3, Fig. 7c) and GFAP-expressing cells (n = 3, Fig. 7d). Furthermore, single channels had a mean amplitude of −2.0 ± 0.1 pA in GFAP-expressing cells (n = 13) and −2.2 ± 0.1 pA in neurons (n = 4) at −80 mV corresponding to a typical GABAAR conductance of 24.5 ± 0.7 pS and 27.7 ± 0.9 pS, respectively (assuming a reversal potential of ∼0 mV in our recording condition). 6/10 of the remaining silent patches from GFAP-expressing cells placed at < 5 μm from a recorded neuroblast displayed single channel activity upon a 5 s-long depolarization of the progenitor from a Vpatch of −60 to +60 mV (using VR of −60 mV10,24). The single channel activity lasted 30 to 60 s and were blocked by gabazine (n = 3). Insets in Fig. 7c-e display single channels at a higher time scale and corresponding amplitude histograms. These data demonstrate that neuroblasts release GABA both spontaneously and upon depolarization, and that released GABA can directly activate GABAARs on neighboring GFAP-expressing cells.
Figure 7.
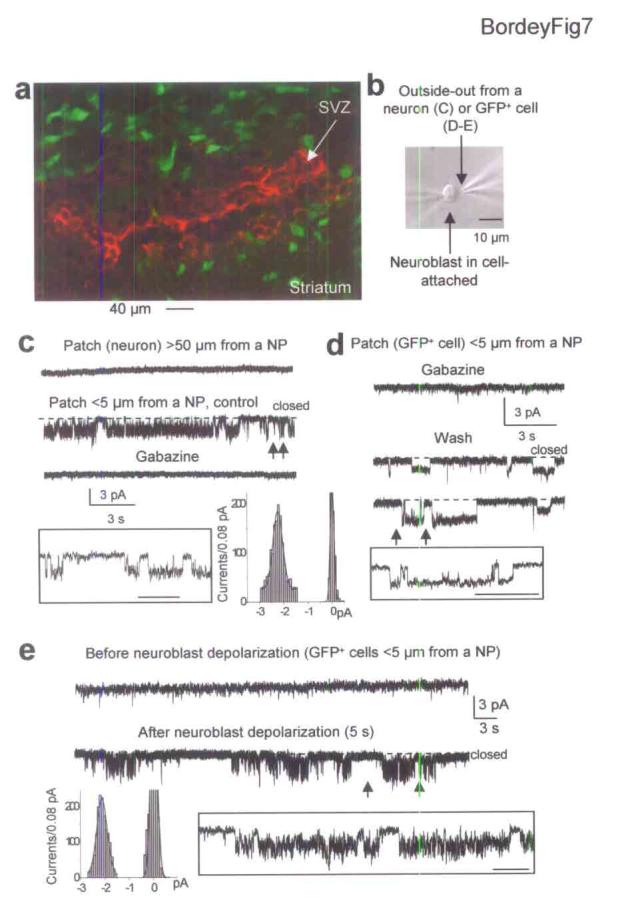
Neuroblasts release GABA spontaneously and upon depolarization. (a) GABA immunostaining (red) in the SVZ of GFAPP-GFP mice. (b) Photograph of a neuroblast recorded in cell-attached above the slice. The second patch electrode contains an outside-out patch from a GFP+ cell (or a striatal neuron as in C) positioned < 5 μm from the simultaneously recorded neuroblast. (c) Simultaneous recordings from a cell-attached neuroblast (Vpip of 0 mV) and an outside-out patch from a striatal neuron held at −80 mV. No single channel activity is detected when the patch is 50 μm away from the neuroblast but channel activity is observed when the patch is < 5 μm from the neuroblast shown in (b) and is blocked by 20 μM gabazine. Insets: Higher time scale of the trace between the arrows and amplitude histogram of middle records. (d-e) Simultaneous recordings from a cell-attached neuroblast and an outside-out patch from a GFP+ cell positioned < 5 μm from the neuroblast. (d) No channel activity is observed when recording with 20 μM gabazine but single channel activity develops upon gabazine removal. (e) Spontaneous channel activity develops after a 5 s-long depolarization of the cell-attached neuroblast (Vpip from 0 to −120 mV). Insets: Traces between the arrows displayed at higher time scale. In (e), amplitude histogram of depolarization-induced channels. Scale bar in insets: 200, 500 and 500 μm in c, d and e, respectively. These experiments were performed in the presence of 20 μM DNQX and 50 μM D-APV but without MFA.
GABAAR activation reduces the proliferation of GFAP cells
We examined whether activation of GABAARs in GFAP-expressing cells influence the proliferation of these cells, as previously shown for embryonic cells14, including SVZ cells13. Pairs of hemisected slices were incubated in the presence of the mitotic marker 5-bromo-2′-deoxyuridine (0.05% BrdU) for 18 hrs either in control conditions (without MFA) or in the presence of BIC (50 μM). After 18 hrs, hemisected slices were fixed and processed for GFP and BrdU immunostaining and confocal z-stacks (10 sections spaced by 1 μm) of 2 to 3 fields of view were taken in control and treated (opposite) hemispheres. Fig. 8a represents a reconstruction of a 10 micron z-stack in control and in bicuculline-treated sections. Inhibition of GABAARs with BIC alone significantly (P < 0.001, unpaired t-test with unequal variance) increased the percentage of GFP-immunopositive cells that stained positive for BrdU from 8.8 ± 1.5% (665 GFP+ cells counted, 95 ± 41 cells, mean ± SEM) to 17.9 ± 1.5% (1064 cells, mean of 142 ± 47 cells, Fig. 8b, 6 pairs of hemisected slices from 5 mice). To prevent an increase in the slice excitability and release of various factors from the slice, these experiments were repeated in the presence of the glutamate receptor blockers DNQX (20 μM) to block AMPA/kainate receptors and D-APV (50 μM) to block NMDA receptors. In this condition, inhibition of GABAARs also significantly (P < 0.001) increased the percentage of GFP-immunopositive cells that stained positive for BrdU from 8.8 ± 1.1% (1643 GFP+ cells, mean of 182 ± 7 cells) to 19.7 ± 1.7% (1238 cells, mean of 137 ± 15 cells, Fig. 8c, 4 pairs of slices, 9 resected sections, 3 mice). We next tested whether the blocker of GAT4 transporters SNAP5114 (100 μM) would limit the number of proliferative GFAP-expressing cells since we previously showed that SNAP5114 increased ambient GABA levels in the SVZ11 (see also Fig. 5). Inhibition of GAT4 transporters with SNAP5114 significantly (P < 0.05) reduced the percentage of GFP-immunopositive cells that stained positive for BrdU from 10.9 ± 1.9% (1909 GFP+ cells, mean of 127 ± 15) to 6.7 ± 1.2% (1979 cells, mean of 116 ± 13, Fig. 8c, 6 pairs of slices, 15 resected sections, 4 mice). These data suggest that GABAAR activation in GFAP-expressing cells by ambient GABA reduces the proliferation of these cells.
Figure 8.
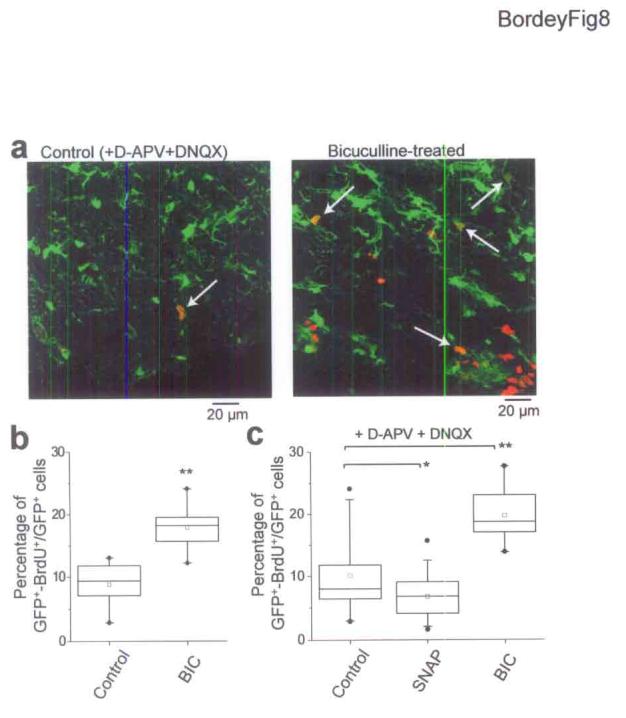
GABAAR activation limits the proliferation of GFAP-expressing cells. (a) Reconstructions of 10 μm z-stacks of BrdU (red) and GFP (green) immunostaining in control and BIC-treated slices. White arrows indicate some GFP+ cells that are BrdU+. The reconstructed stack in the BIC-treated slice included a region outside the SVZ. BrdU+ cells were within the SVZ. (b) Percentage of GFP+ cells that are BrdU+ in control and in 50 μM BIC-treated slices. (c) Percentage of GFP+ cells that are BrdU+ in control in the presence of AMPA/kainate and NMDA receptor blockers (20 μM DNQX and 50 μM D-APV, respectively) and in 50 μM BIC- or 100 μM SNAP5114 (SNAP)-treated slices. SNAP-5114 is a non-transportable blocker of GAT4 transporters. *: P < 0.05, **: P < 0.001 (unpaired t-test with unequal variance), box: 25th and 75th percentiles, upper and lower bars: 5th and 95th percentiles, •: minimum and maximum, □: mean, middle bar: median.
DISCUSSION
We demonstrate here that nonsynaptically, SNARE-independent released GABA from neuroblasts activates GABAARs in GFAP-expressing cells of the SVZ, thought to be stem cells6-8, and that tonic GABAAR activation in GFAP-expressing cells reduces their proliferation.
Nonsynaptic GABAergic signaling between SVZ progenitors
Recorded cells were identified as GFAP-expressing cells prior to recording by direct visualization of fluorescence in GFAPP-GFP mice21. Recorded GFP+ cells had passive membrane properties similar to those of astrocytes but displayed a less elaborated morphology22. In addition, recorded GFP+ cells stained positive for nestin, a marker of immature cells but not astrocytes1,25. GABA currents in GFAP-expressing cells were blocked by several GABAAR antagonists and were carried by Cl− ions, identifying these currents as GABAAR-mediated responses. Electrical or high K+ stimulations of SVZ progenitors induced nonsynaptic GABAA currents in GFAP-expressing cells, suggesting that SVZ progenitors released GABA. Evoked currents developed slowly and decayed over several seconds. This time course is significantly slower than that of synaptic currents. The four following results support nonsynaptic GABA release. (1) Synapsin I staining was absent in the SVZ, (2) The kinetics course and amplitude of evoked GABAA currents were dependent on the distance between stimulating electrodes and recorded cells. (3) Blockade of action potentials or voltage-gated Ca2+ channels, or removal of extracellular Ca2+ did not prevent evoked GABAA currents. (4) α-latrotoxin, which stimulates vesicular exocytosis from presynaptic terminals28, did not induce any synaptic currents in GFAP-expressing cells. The persistence of evoked GABAA currents in GFAP-expressing cells following BoNT treatments suggests that GABA release is independent of a classical SNARE complex. Taken together, these results demonstrate that GABA is nonsynaptically, SNARE-independently released in the postnatal mammalian SVZ. Such nonsynaptic, Q-SNARE-independent GABAergic communication has been observed in embryonic and neonatal hippocampal neurons19. However, in contrast with GABAergic signaling in the neonatal hippocampus, we found that GATs control ambient GABA levels and limit the diffusion of released GABA. GAT3/4, and to a lesser extent GAT1 transporters, played a major role in regulating ambient GABA levels in the SVZ.
GABA is present in neuroblasts11 but not in GFAP-expressing cells. We cannot rule out that GABA is also contained in glioblasts but this population is a minority at the ages studied11,32,33. It was previously reported by us and others that SVZ neuroblasts express glutamic acid decarboxylase10,34,35. Neuroblasts displayed spontaneous depolarizations and our paired recordings indicate that neuroblasts release GABA spontaneously and upon depolarization. Collectively these data strongly suggest that GABA is spontaneously released from neuroblasts, diffuses and activates GABAARs on neighboring GFAP-expressing cells known to ensheath neuroblasts1. While further studies are required to define the mechanisms of GABA release from neuroblasts, the two following mechanisms can be ruled out: (1) GABA transport reversal although release via GAT2 transport reversal cannot be ruled out; (2) Release through hemichannels as observed for ATP and glutamate36,37 since MFA is a blocker of hemichannels26. One possible mechanism of GABA release is via unconventional exocytosis triggered by Ca2+ release from intracellular stores as buffering intracellular Ca2+ prevented the induction of evoked GABAA currents. Furthermore, neuroblasts display spontaneous Ca2+ transients (Huang F and Bordey A, unpublished observations), contain synapsin III, a protein associated with synaptic vesicles38,39.
Function of GABAergic signaling between SVZ progenitors
Our data indicate that in the SVZ neuroblasts communicate with GFAP-expressing cells by releasing GABA that activates GABAARs in GFAP-expressing cells (Supplemental Fig. 3). Furthermore, we show that GABAAR activation in GFAP-expressing cells limits their progression through the cell cycle. As more neuroblasts are generated, it is possible that more GABA is released in the extracellular space resulting in increased ambient GABA levels and GABAAR activation in GFAP-expressing cells. Because a subset of GFAP-expressing cells are thought to generate neuroblasts6-8, increase in the number of neuroblasts serves as a negative feedback to decrease GFAP-expressing cell proliferation and neuroblast production by activating GABAARs. This is consistent with earlier studies showing that GABA alters proliferation14,15,18, and in particular reduces the proliferation of embryonic SVZ cells13, which are thought to be lineally related to GFAP-expressing cells in the postnatal SVZ40. Thus, with respect to ambient GABA's proliferative action, there is a nice parallel between results from embryonic and postnatal SVZ cells. Such a feedback mechanism may also apply for glioblast production from GFAP-expressing cells or radial glia in the neonatal SVZ assuming that glioblasts contain GABA32,33,41. Furthermore, the feedback mechanism provided by neuroblasts on GFAP-expressing cell proliferation fits well with the constant migration of neuroblasts away from the SVZ to the olfactory bulb5,42,43, which would limit ambient GABA accumulation. This feedback also fits well with the increased proliferation of GFAP-expressing cells following elimination of neuroblasts9. In conclusion, changes in GABA levels due to neuroblast migration or production provide a mechanism to control the proliferation of GFAP-expressing cells and maintain a balance between neuroblast production and mobilization.
METHODS
Slice preparation and electrophysiology
Coronal (250 μm-thick) brain slices from 16- to 41-day old transgenic mice (Jackson laboratory)21 were prepared as previously described10,24. Experimental procedures were in accordance with the animal welfare guidelines of Yale University. The standard external solution contained (in mM): NaCl 125, KCl 2.5, CaCl2 2, MgCl2 1, NaH2PO4 1.25, NaHCO3 25, glucose 10, pH 7.4 when equilibrated with 95% O2/5% CO2. Whole-cell and gramicidin perforated patch-clamp recordings were obtained as previously described10,22,44 and as detailed in Supplementary Method. GFAP-expressing cells had a mean capacitance of 17.7 ± 0.5 pF (n = 111) with 100 μM MFA. Electrical stimulation was performed using a 6-8 MΩ patch pipette (tip diameter < 1 μm) filled with ACSF and surrounded with a silver wire. For BoNT treatment, acutely prepared slices were transferred to tissue culture inserts (0.4 μm-membrane, Becton Dickinson) in 35 mm 6-well plates, which contained external solution (1 ml/well) with antibiotics, and with or without 100 ng/ml BoNT/A or BoNT/B for 18 hrs at 37°C in a 95% O2/5% CO2-saturated atmosphere.
Immunohistochemistry and proliferation assay
Slices were fixed for 3 hr in 4% paraformaldehyde (PAF) in PBS containing 4% sucrose (PBSS). Immunostaining was performed as previously described10,11. The primary antibodies included mouse anti-nestin IgG1 antibody (1:1, Rat-401, Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa) and rabbit anti-GABA antibody (1:500, Sigma), mouse Alexa 594-conjugated anti-BrdU IgG1 antibody (1:1000, Molecular Probes) and rabbit anti-GFP antibody (1:200, Abcam). Secondary antibodies were the appropriate Alexa Fluor (1:1000, Molecular Probes). Stained sections were viewed on a confocal scanning laser microscope (Biorad MRC600) or on an epifluorescence microscope (Olympus BX51) using standard procedures. Proliferation experiments were exactly as previously described45 and as detailed in Supplementary Method.
Data analysis
Data acquisition and analysis were performed using PClamp 8 (Axon Instruments, CA). For analysis of cell proliferation, confocal z-stacks (10 sections spaced by 1 μm) were obtained in 2-3 fields of the proximal RMS or anterior SVZ per slice using a LSM 510 META NLO confocal microscope (Carl Zeiss, Inc). The number of GFP+ cells per field and then the number of cells that were both GFP+ and BrdU+ were counted using Adobe Photoshop. None of the control proliferation data (for SNAP5114 and the bicuculline experiments) were significantly different. We combined the two control data for SNAP5114 and bicuculline that were performed with glutamate receptor blockers. Data are given as mean ± SEM, n being the number of cells or slices when noted. Levels of significance were determined by Student's t-test (Statview, Inc).
Supplementary Material
ACKNOWLEDMENTS
We thank C. Broberger, J. Fitzpatrick, U. Misgeld, M. Sarkisian, S. Titz and A. Williamson for valuable comments on the manuscript. We thank C.A. Greer for providing us with a scanning confocal microscope. This work was supported by a grant from the National Multiple Sclerosis Society (A.B.) and the National Institute of Health (NS042189 and NS048256; A.B.).
REFERENCES
- 1.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 3.Luskin MB, Zigova T, Soteres BJ, Stewart RR. Neuronal progenitor cells derived from the anterior subventricular zone of the neonatal rat forebrain continue to proliferate in vitro and express a neuronal phenotype. Mol. Cell Neurosci. 1997;8:351–366. doi: 10.1006/mcne.1996.0592. [DOI] [PubMed] [Google Scholar]
- 4.Belluzzi O, Benedusi M, Ackman J, LoTurco JJ. Electrophysiological differentiation of new neurons in the olfactory bulb. J. Neurosci. 2003;23:10411–10418. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat. Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- 6.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 7.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 8.Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 9.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc. Natl. Acad. Sci. U. S. A. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang DD, Krueger DD, Bordey A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J. Physiol (Lond) 2003;550:785–800. doi: 10.1113/jphysiol.2003.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolteus AJ, Bordey A. GABA Release and Uptake Regulate Neuronal Precursor Migration in the Postnatal Subventricular Zone. J. Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fueshko SM, Key S, Wray S. GABA inhibits migration of luteinizing hormone-releasing hormone neurons in embryonic olfactory explants. J. Neurosci. 1998;18:2560–2569. doi: 10.1523/JNEUROSCI.18-07-02560.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haydar TF, Wang F, Schwartz ML, Rakic P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J. Neurosci. 2000;20:5764–5774. doi: 10.1523/JNEUROSCI.20-15-05764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 15.Barker JL, et al. GABAergic cells and signals in CNS development. Perspect. Dev. Neurobiol. 1998;5:305–322. [PubMed] [Google Scholar]
- 16.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen L, et al. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001;305:187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen L, et al. Autocrine/paracrine activation of the GABA(A) receptor inhibits the proliferation of neurogenic polysialylated neural cell adhesion molecule-positive (PSA-NCAM+) precursor cells from postnatal striatum. J. Neurosci. 2003;23:3278–3294. doi: 10.1523/JNEUROSCI.23-08-03278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demarque M, et al. Paracrine intercellular communication by a Ca2+− and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002;36:1051–1061. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- 20.Verderio C, et al. SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron. 2004;41:599–610. doi: 10.1016/s0896-6273(04)00077-7. [DOI] [PubMed] [Google Scholar]
- 21.Zhuo L, et al. Live astrocytes visualized by green fluorescent protein in transgenic mice. Dev. Biol. 1997;187:36–42. doi: 10.1006/dbio.1997.8601. [DOI] [PubMed] [Google Scholar]
- 22.Bordey A, Sontheimer H. Ion channel expression by astrocytes in situ: comparison of different CNS regions. Glia. 2000;30:27–38. doi: 10.1002/(sici)1098-1136(200003)30:1<27::aid-glia4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Noctor SC, et al. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J. Neurosci. 2002;22:3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang DD, Krueger DD, Bordey A. Biophysical properties and ionic signature of neuronal progenitors of the postnatal subventricular zone in situ. J. Neurophysiol. 2003;90:2291–2302. doi: 10.1152/jn.01116.2002. [DOI] [PubMed] [Google Scholar]
- 25.Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J. Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harks EG, et al. Fenamates: a novel class of reversible gap junction blockers. J. Pharmacol. Exp. Ther. 2001;298:1033–1041. [PubMed] [Google Scholar]
- 27.Smith AJ, Oxley B, Malpas S, Pillai GV, Simpson PB. Compounds exhibiting selective efficacy for different {beta} subunits of human recombinant GABAA receptors. J. Pharmacol. Exp. Ther. 2004 doi: 10.1124/jpet.104.070342. [DOI] [PubMed] [Google Scholar]
- 28.Sudhof TC. alpha-Latrotoxin and its receptors: neurexins and CIRL/latrophilins. Annu. Rev. Neurosci. 2001;24:933–962. doi: 10.1146/annurev.neuro.24.1.933. [DOI] [PubMed] [Google Scholar]
- 29.Hilfiker S, et al. Synapsins as regulators of neurotransmitter release. Philos. Trans. R. Soc. Lond B Biol. Sci. 1999;354:269–279. doi: 10.1098/rstb.1999.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem. Int. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Corcuera B, Liu QR, Mandiyan S, Nelson H, Nelson N. Expression of a mouse brain cDNA encoding novel gamma- aminobutyric acid transporter. J. Biol. Chem. 1992;267:17491–17493. [PubMed] [Google Scholar]
- 32.Levison SW, Goldman JE. Multipotential and lineage restricted precursors coexist in the mammalian perinatal subventricular zone. J. Neurosci. Res. 1997;48:83–94. [PubMed] [Google Scholar]
- 33.Levison SW, Chuang C, Abramson BJ, Goldman JE. The migrational patterns and developmental fates of glial precursors in the rat subventricular zone are temporally regulated. Development. 1993;119:611–622. doi: 10.1242/dev.119.3.611. [DOI] [PubMed] [Google Scholar]
- 34.De Marchis S, et al. GABAergic phenotypic differentiation of a subpopulation of subventricular derived migrating progenitors. Eur. J. Neurosci. 2004;20:1307–1317. doi: 10.1111/j.1460-9568.2004.03584.x. [DOI] [PubMed] [Google Scholar]
- 35.Stewart RR, Hoge GJ, Zigova T, Luskin MB. Neural progenitor cells of the neonatal rat anterior subventricular zone express functional GABA(A) receptors. J. Neurobiol. 2002;50:305–322. doi: 10.1002/neu.10038. [DOI] [PubMed] [Google Scholar]
- 36.Cotrina ML, et al. Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J. Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pieribone VA, et al. Expression of synapsin III in nerve terminals and neurogenic regions of the adult brain. J. Comp Neurol. 2002;454:105–114. doi: 10.1002/cne.10417. [DOI] [PubMed] [Google Scholar]
- 39.Kao HT, et al. A third member of the synapsin gene family. Proc. Natl. Acad. Sci. U. S. A. 1998;95:4667–4672. doi: 10.1073/pnas.95.8.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tramontin AD, Garcia-Verdugo JM, Lim DA, Alvarez-Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb. Cortex. 2003;13:580–587. doi: 10.1093/cercor/13.6.580. [DOI] [PubMed] [Google Scholar]
- 41.Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- 42.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 43.Luskin MB, Boone MS. Rate and pattern of migration of lineally-related olfactory bulb interneurons generated postnatally in the subventricular zone of the rat. Chem. Senses. 1994;19:695–714. doi: 10.1093/chemse/19.6.695. [DOI] [PubMed] [Google Scholar]
- 44.Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pfluegers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- 45.Haydar TF, Bambrick LL, Krueger BK, Rakic P. Organotypic slice cultures for analysis of proliferation, cell death, and migration in the embryonic neocortex. Brain Res. Brain Res. Protoc. 1999;4:425–437. doi: 10.1016/s1385-299x(99)00033-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


