Abstract
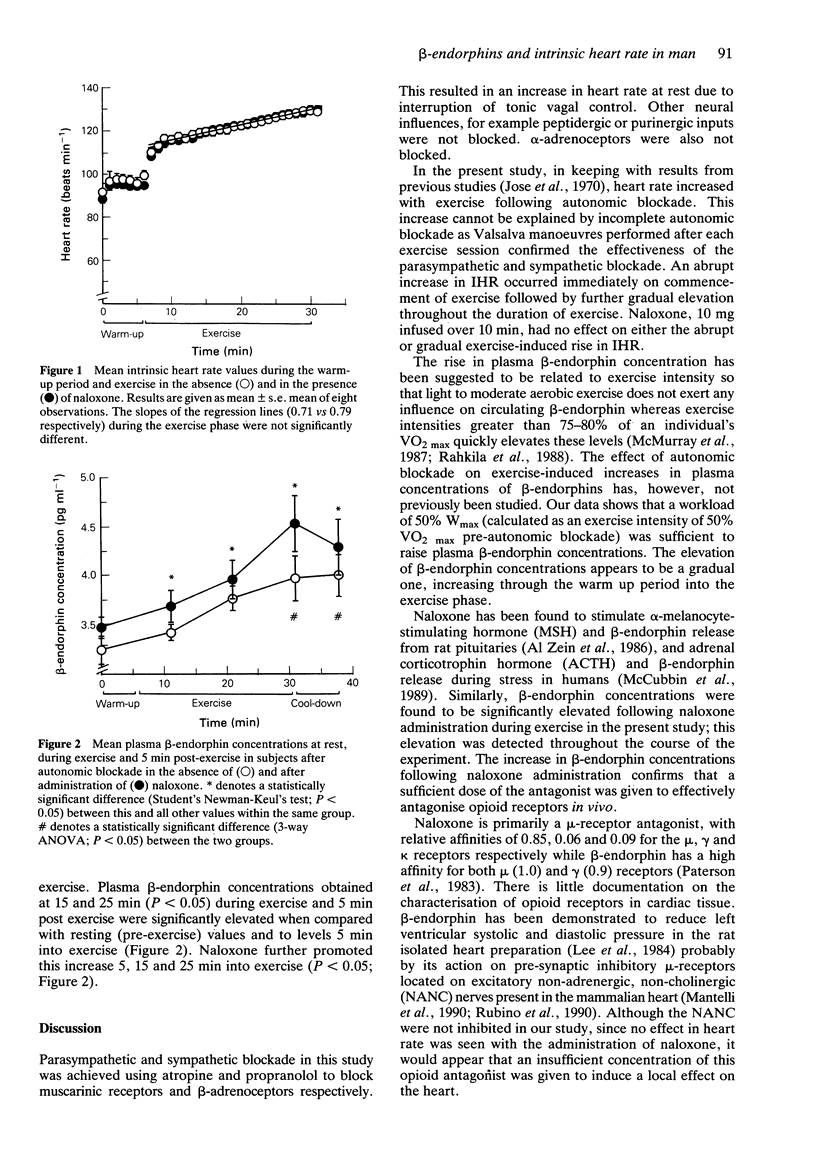
1. Intrinsic heart rate (IHR: heart rate following autonomic blockade with atropine and propranolol) increases with exercise. The opioid antagonist naloxone has been shown to decrease IHR at rest, raising the possibility that increases in IHR with exercise are beta-endorphin related, since beta-endorphin concentrations have also been shown to rise during exercise. 2. We examined the effects of naloxone (10 mg) on IHR and plasma beta-endorphin levels during aerobic exercise in eight healthy, male subjects in a single blind, crossover study. 3. IHR increased with 25 min bicycling from 97.1 +/- 1.4 to 129.7 +/- 1.2 beats min-1 (mean +/- s.e. mean). This rise was not affected by administration of naloxone. 4. Plasma beta-endorphin concentration rose from 31.1 +/- 3.8 to 94.9 +/- 23.9 pg ml-1 after 25 min exercise. This exercise-induced rise in beta-endorphin concentration was further increased (P less than 0.05) in the presence of naloxone. 5. Our results confirm a rise in IHR and beta-endorphin concentrations with acute exercise but indicate that the changes in IHR are not endorphin-related.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al Zein M., Lutz-Bucher B., Koch B. Modulation by Leu-enkephalin of peptide release from perifused neurointermediate pituitary. II. Inhibition of calcium-mediated secretion of alpha-MSH and beta-endorphin. Neuroendocrinology. 1986;42(3):248–254. doi: 10.1159/000124447. [DOI] [PubMed] [Google Scholar]
- Bramnert M. The effect of naloxone on blood pressure, heart rate, plasma catecholamines, renin activity and aldosterone following exercise in healthy males. Regul Pept. 1988 Sep;22(4):295–301. doi: 10.1016/0167-0115(88)90106-1. [DOI] [PubMed] [Google Scholar]
- Brooks S., Burrin J., Cheetham M. E., Hall G. M., Yeo T., Williams C. The responses of the catecholamines and beta-endorphin to brief maximal exercise in man. Eur J Appl Physiol Occup Physiol. 1988;57(2):230–234. doi: 10.1007/BF00640668. [DOI] [PubMed] [Google Scholar]
- Engler D., Pham T., Fullerton M. J., Funder J. W., Clarke I. J. Studies of the regulation of the hypothalamic-pituitary-adrenal axis in sheep with hypothalamic-pituitary disconnection. I. Effect of an audiovisual stimulus and insulin-induced hypoglycemia. Neuroendocrinology. 1988 Nov;48(5):551–560. doi: 10.1159/000125062. [DOI] [PubMed] [Google Scholar]
- Jennings G. L., Bobik A., Fagan E. T., Korner P. I. Pindolol pharmacokinetics in relation to time course of inhibition of exercise tachycardia. Br J Clin Pharmacol. 1979 Mar;7(3):245–256. doi: 10.1111/j.1365-2125.1979.tb00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose A. D., Stitt F., Collison D. The effects of exercise and changes in body temperature on the intrinsic heart rate in man. Am Heart J. 1970 Apr;79(4):488–498. doi: 10.1016/0002-8703(70)90254-1. [DOI] [PubMed] [Google Scholar]
- Lee A. Y., Zhan C. Y., Wong T. M. Effects of beta-endorphin on the contraction and electrical activity of the isolated perfused rat heart. Int J Pept Protein Res. 1984 Nov;24(5):525–528. doi: 10.1111/j.1399-3011.1984.tb03154.x. [DOI] [PubMed] [Google Scholar]
- Mantelli L., Amerini S., Rubino A., Ledda F. Characterization of opioid receptors modulating the function of capsaicin-sensitive neurons in guinea-pig atria. Eur J Pharmacol. 1990 May 16;180(2-3):325–330. doi: 10.1016/0014-2999(90)90317-y. [DOI] [PubMed] [Google Scholar]
- McCubbin J. A., Surwit R. S., Williams R. B., Nemeroff C. B., McNeilly M. Altered pituitary hormone response to naloxone in hypertension development. Hypertension. 1989 Dec;14(6):636–644. doi: 10.1161/01.hyp.14.6.636. [DOI] [PubMed] [Google Scholar]
- McMurray R. G., Forsythe W. A., Mar M. H., Hardy C. J. Exercise intensity-related responses of beta-endorphin and catecholamines. Med Sci Sports Exerc. 1987 Dec;19(6):570–574. [PubMed] [Google Scholar]
- PICKERING G. Regulation of body temperature in health and disease. Lancet. 1958 Jan 4;1(7010):1–cont'd. doi: 10.1016/s0140-6736(58)92512-1. [DOI] [PubMed] [Google Scholar]
- Paterson S. J., Robson L. E., Kosterlitz H. W. Classification of opioid receptors. Br Med Bull. 1983 Jan;39(1):31–36. doi: 10.1093/oxfordjournals.bmb.a071787. [DOI] [PubMed] [Google Scholar]
- Rahkila P., Hakala E., Alén M., Salminen K., Laatikainen T. Beta-endorphin and corticotropin release is dependent on a threshold intensity of running exercise in male endurance athletes. Life Sci. 1988;43(6):551–558. doi: 10.1016/0024-3205(88)90158-0. [DOI] [PubMed] [Google Scholar]
- Rees L. H., Cook D. M., Kendall J. W., Allen C. F., Kramer R. M., Ratcliffe J. G., Knight R. A. A radioimmunoassay for rat plasma ACTH. Endocrinology. 1971 Jul;89(1):254–261. doi: 10.1210/endo-89-1-254. [DOI] [PubMed] [Google Scholar]
- Rubino A., Mantelli L., Amerini S., Ledda F. Characterization of opioid receptors involved in the modulation of NANC neurotransmission in the mammalian heart. Acta Physiol Hung. 1990;75 (Suppl):251–252. [PubMed] [Google Scholar]
- Staessen J., Fiocchi R., Bouillon R., Fagard R., Hespel P., Lijnen P., Moerman E., Amery A. Effects of opioid antagonism on the haemodynamic and hormonal responses to exercise. Clin Sci (Lond) 1988 Sep;75(3):293–300. doi: 10.1042/cs0750293. [DOI] [PubMed] [Google Scholar]


