Abstract
1. A gamma scintigraphic study has been carried out on the precorneal residence and pharmacodynamic action of a radiolabelled New Ophthalmic Delivery System (NODS) containing pilocarpine nitrate in 12 healthy volunteers. 2. The NODS was radiolabelled with the soluble marker technetium-99m labelled diethylenetriaminepentaacetic acid, to mark the release characteristics of soluble drugs contained within the matrix. 3. The relationship between the precorneal residence time of the marker and the duration of drug effect on intraocular pressure and pupil diameter was monitored. Results obtained following administration of the NODS were compared with those obtained after administration of a 25 microliters drop of a 2% w/v pilocarpine nitrate solution. Each formulation was administered to one eye only, the other eye acting as a control. 4. Dissolution of the radiolabel from the NODS in vivo showed considerable intersubject variation with half-times of dissolution ranging from 46 s to 833 s (mean +/- s.d. -280 +/- 217 s), the mean (+/- s.d.) half-time of clearance of the radiolabel from the NODS and corneal region of interest was 406 +/- 214 s whereas the radiolabelled solution had a mean (+/- s.d.) ocular surface residence time of 2.9 +/- 1.5 s. 5. Pupil diameter and intraocular pressure were measured for 5 h post-administration of the NODS and the solution. After both treatments pupil diameter was significantly constricted in the test eye when compared with the control eye (P less than 0.001; Student's paired t-test). Pupil diameter was constricted by 52% after administration of the NODS and by 35% after administration of the solution.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



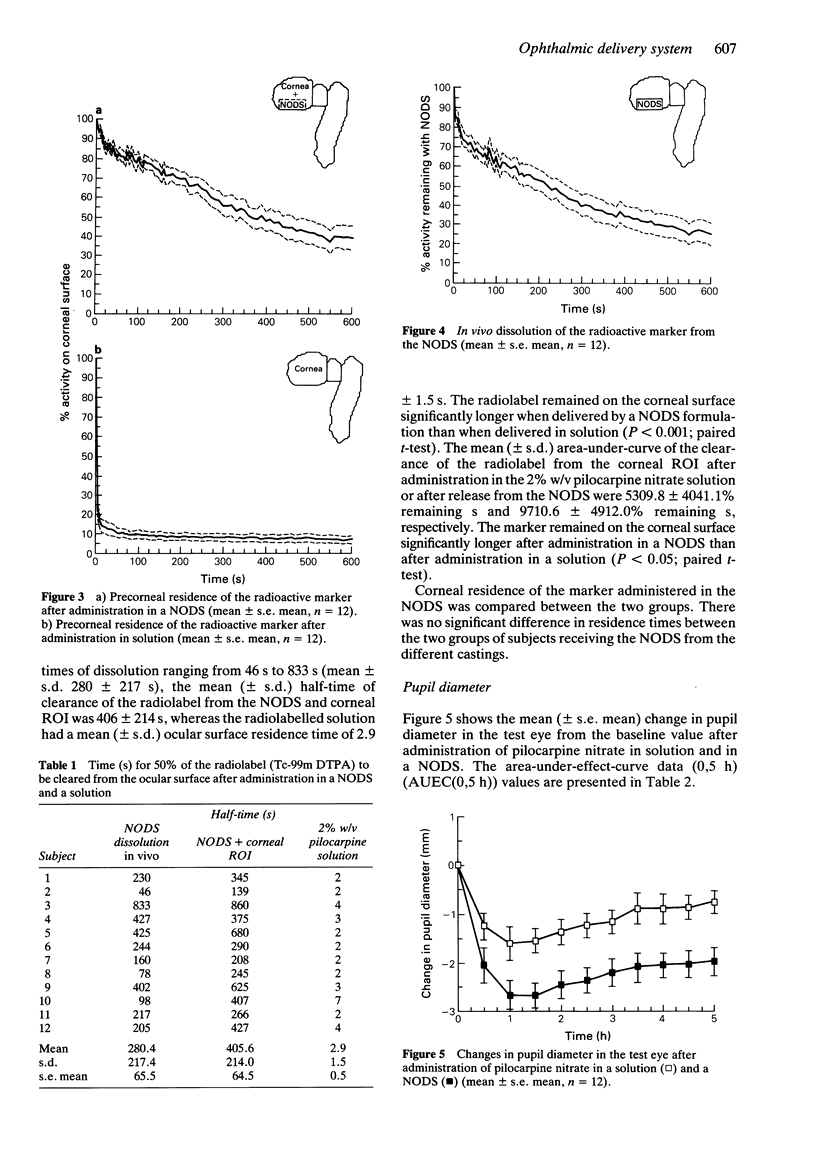
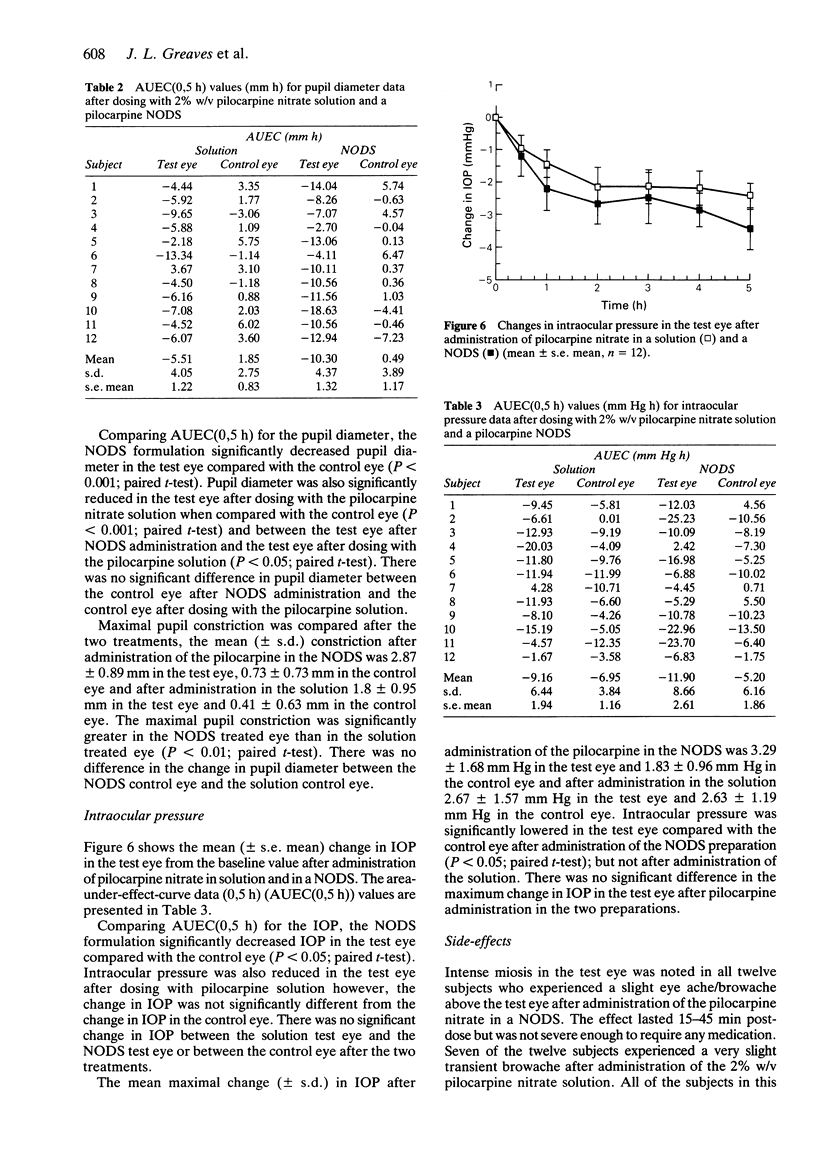

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Grolman B. A new tonometer system. Am J Optom Arch Am Acad Optom. 1972 Aug;49(8):646–660. doi: 10.1097/00006324-197208000-00005. [DOI] [PubMed] [Google Scholar]
- Kelly J. A., Molyneux P. D., Smith S. A., Smith S. E. Relative bioavailability of pilocarpine from a novel ophthalmic delivery system and conventional eyedrop formulations. Br J Ophthalmol. 1989 May;73(5):360–362. doi: 10.1136/bjo.73.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton T. F., Robinson J. R. Ocular evaluation of polyvinyl alcohol vehicle in rabbits. J Pharm Sci. 1975 Aug;64(8):1312–1316. doi: 10.1002/jps.2600640811. [DOI] [PubMed] [Google Scholar]
- Shell J. W., Baker R. W. Diffusional systems for controlled release of drugs to the eye. Ann Ophthalmol. 1974 Oct;6(10):1037-43, 1045. [PubMed] [Google Scholar]
- Shields M. B. The non-contact tonometer. Its value and limitations. Surv Ophthalmol. 1980 Jan-Feb;24(4):211–219. doi: 10.1016/0039-6257(80)90042-9. [DOI] [PubMed] [Google Scholar]
- Yoshioka S., Aso Y., Shibazaki T., Uchiyama M. Stability of pilocarpine ophthalmic formulations. Chem Pharm Bull (Tokyo) 1986 Oct;34(10):4280–4286. doi: 10.1248/cpb.34.4280. [DOI] [PubMed] [Google Scholar]