Abstract
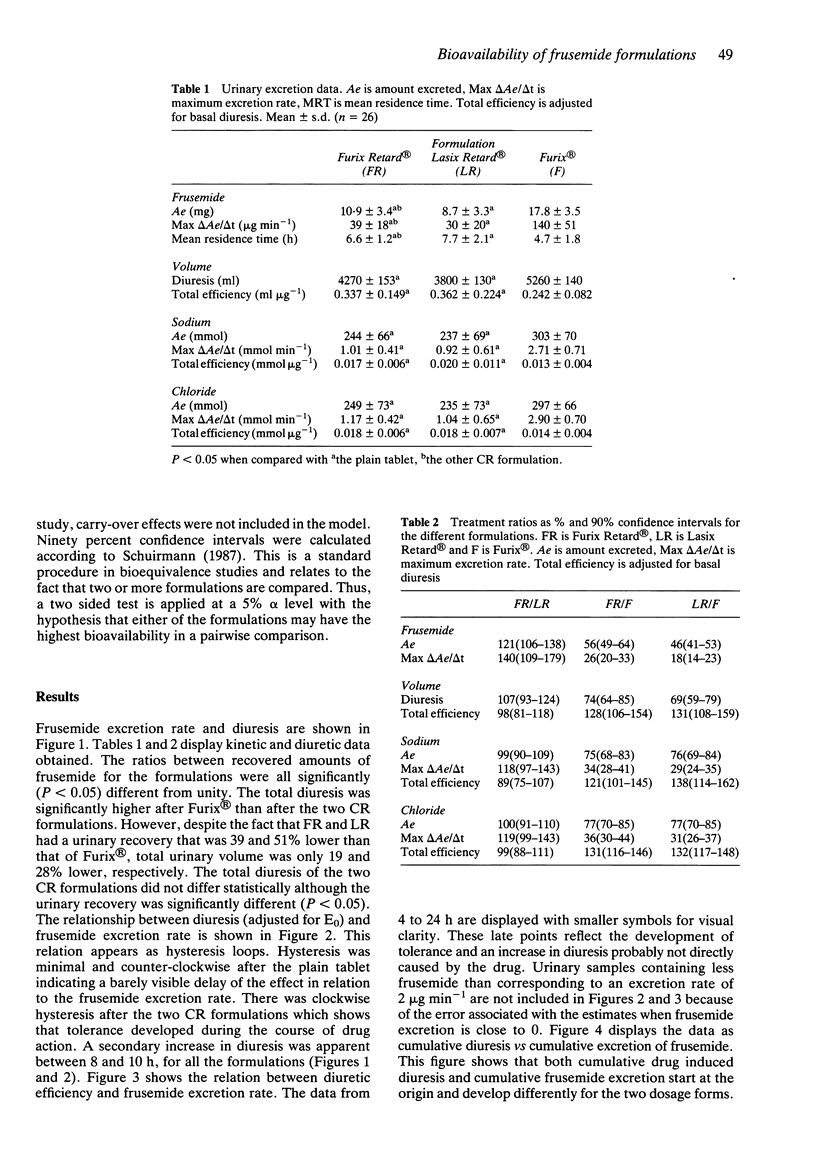
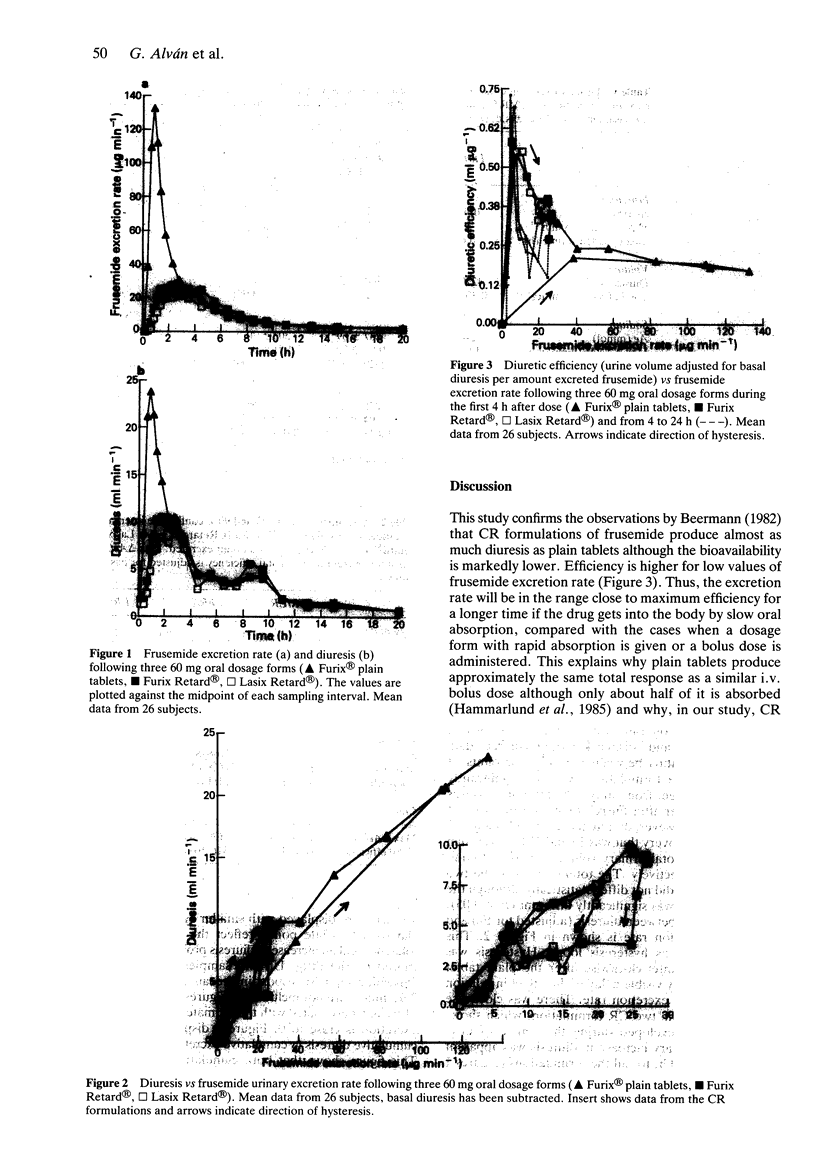
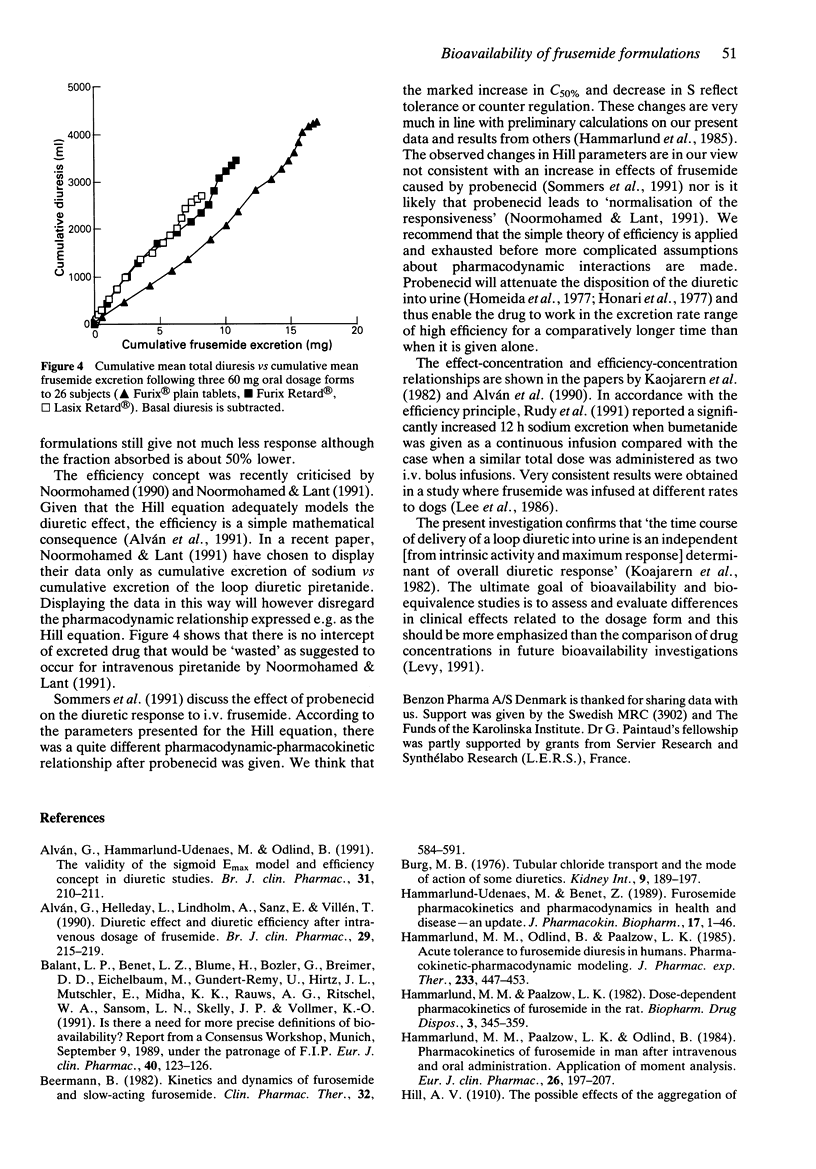
1. Frusemide was given at a dose of 60 mg as two oral controlled release (CR) formulations and as plain tablets in a randomised, balanced, three way cross over design to 26 healthy volunteers. Urinary volume, and contents of frusemide, sodium, chloride and potassium were measured in samples taken over 24 h. 2. There was a marked difference between the CR formulations on one hand and the plain tablets on the other, in excretion of frusemide and diuresis vs time. The total diuretic/saluretic effect was only marginally lower (19 and 28% respectively, P less than 0.05) after CR compared with plain tablets although the fraction absorbed was markedly decreased (39 and 51% lower, respectively, P less than 0.05), estimated as urinary recovery of frusemide. The total diuresis of the two CR formulations did not differ although the urinary recovery was significantly different (P less than 0.05). 3. The diuretic effect vs frusemide excretion rate showed minimal counter-clockwise hysteresis after plain tablets while the CR formulations produced clockwise hysteresis indicating tolerance. 4. In agreement with the concept of efficiency, the higher diuretic/saluretic effect per amount of excreted frusemide may be a consequence of the slower output of frusemide in urine with the CR formulations compared with plain tablets. The major part of the pharmacological effect was produced with a higher efficiency after CR compared with plain tablets. It should be noted that the pharmacokinetics of a drug and its pharmacodynamic potency independently determine the total response.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alván G., Hammarlund-Udenaes M., Odlind B. The validity of the sigmoid Emax model and efficiency concept in diuretic studies. Br J Clin Pharmacol. 1991 Feb;31(2):210–211. doi: 10.1111/j.1365-2125.1991.tb05520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alván G., Helleday L., Lindholm A., Sanz E., Villén T. Diuretic effect and diuretic efficiency after intravenous dosage of frusemide. Br J Clin Pharmacol. 1990 Feb;29(2):215–219. doi: 10.1111/j.1365-2125.1990.tb03622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beermann B. Kinetics and dynamics of furosemide and slow-acting furosemide. Clin Pharmacol Ther. 1982 Nov;32(5):584–591. doi: 10.1038/clpt.1982.207. [DOI] [PubMed] [Google Scholar]
- Burg M. B. Tubular chloride transport and the mode of action of some diuretics. Kidney Int. 1976 Feb;9(2):189–197. doi: 10.1038/ki.1976.20. [DOI] [PubMed] [Google Scholar]
- Hammarlund-Udenaes M., Benet L. Z. Furosemide pharmacokinetics and pharmacodynamics in health and disease--an update. J Pharmacokinet Biopharm. 1989 Feb;17(1):1–46. doi: 10.1007/BF01059086. [DOI] [PubMed] [Google Scholar]
- Hammarlund M. M., Odlind B., Paalzow L. K. Acute tolerance to furosemide diuresis in humans. Pharmacokinetic-pharmacodynamic modeling. J Pharmacol Exp Ther. 1985 May;233(2):447–453. [PubMed] [Google Scholar]
- Hammarlund M. M., Paalzow L. K. Dose-dependent pharmacokinetics of furosemide in the rat. Biopharm Drug Dispos. 1982 Oct-Dec;3(4):345–359. doi: 10.1002/bdd.2510030408. [DOI] [PubMed] [Google Scholar]
- Hammarlund M. M., Paalzow L. K., Odlind B. Pharmacokinetics of furosemide in man after intravenous and oral administration. Application of moment analysis. Eur J Clin Pharmacol. 1984;26(2):197–207. doi: 10.1007/BF00630286. [DOI] [PubMed] [Google Scholar]
- Holford N. H., Sheiner L. B. Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin Pharmacokinet. 1981 Nov-Dec;6(6):429–453. doi: 10.2165/00003088-198106060-00002. [DOI] [PubMed] [Google Scholar]
- Homeida M., Roberts C., Branch R. A. Influence of probenecid and spironolactone on furosemide kinetics and dynamics in man. Clin Pharmacol Ther. 1977 Oct;22(4):402–409. doi: 10.1002/cpt1977224402. [DOI] [PubMed] [Google Scholar]
- Honari J., Blair A. D., Cutler R. E. Effects of probenecid on furosemide kinetics and natriuresis in man. Clin Pharmacol Ther. 1977 Oct;22(4):395–401. doi: 10.1002/cpt1977224395. [DOI] [PubMed] [Google Scholar]
- Kaojarern S., Day B., Brater D. C. The time course of delivery of furosemide into urine: an independent determinant of overall response. Kidney Int. 1982 Jul;22(1):69–74. doi: 10.1038/ki.1982.134. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Li T., Chiou W. L. Effect of intravenous infusion time on the pharmacokinetics and pharmacodynamics of the same total dose of furosemide. Biopharm Drug Dispos. 1986 Nov-Dec;7(6):537–547. doi: 10.1002/bdd.2510070603. [DOI] [PubMed] [Google Scholar]
- Noormohamed F. H., Lant A. F. Analysis of the natriuretic action of a loop diuretic, piretanide, in man. Br J Clin Pharmacol. 1991 Apr;31(4):463–469. doi: 10.1111/j.1365-2125.1991.tb05563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noormohamed F. H. Use of Emax model in diuretic studies. Br J Clin Pharmacol. 1990 Dec;30(6):907–908. doi: 10.1111/j.1365-2125.1990.tb05460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odlind B., Beermann B. Renal tubular secretion and effects of furosemide. Clin Pharmacol Ther. 1980 Jun;27(6):784–790. doi: 10.1038/clpt.1980.111. [DOI] [PubMed] [Google Scholar]
- Odlind B. Relationship between tubular secretion of furosemide and its saluretic effect. J Pharmacol Exp Ther. 1979 Mar;208(3):515–521. [PubMed] [Google Scholar]
- Rudy D. W., Voelker J. R., Greene P. K., Esparza F. A., Brater D. C. Loop diuretics for chronic renal insufficiency: a continuous infusion is more efficacious than bolus therapy. Ann Intern Med. 1991 Sep 1;115(5):360–366. doi: 10.7326/0003-4819-115-5-360. [DOI] [PubMed] [Google Scholar]
- Schuirmann D. J. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987 Dec;15(6):657–680. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- Sommers D. K., Meyer E. C., Moncrieff J. The influence of co-administered organic acids on the kinetics and dynamics of frusemide. Br J Clin Pharmacol. 1991 Oct;32(4):489–493. doi: 10.1111/j.1365-2125.1991.tb03936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


