Abstract
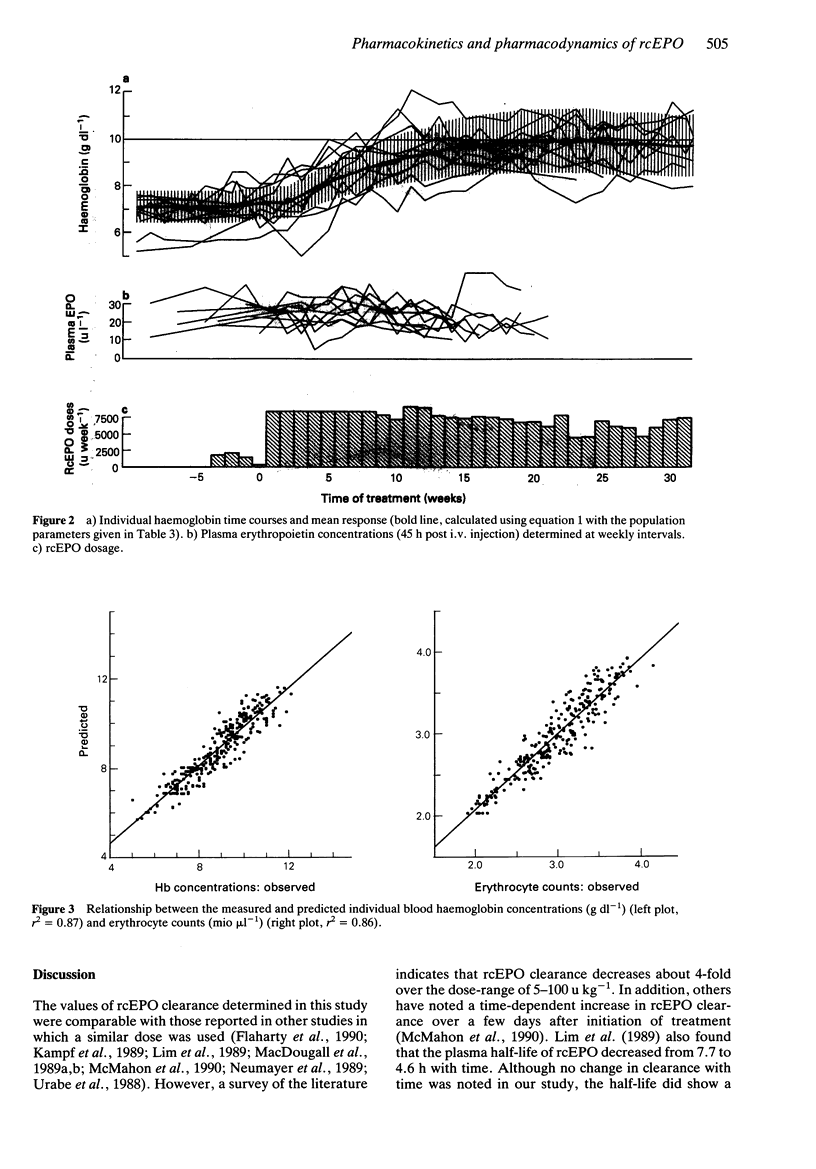
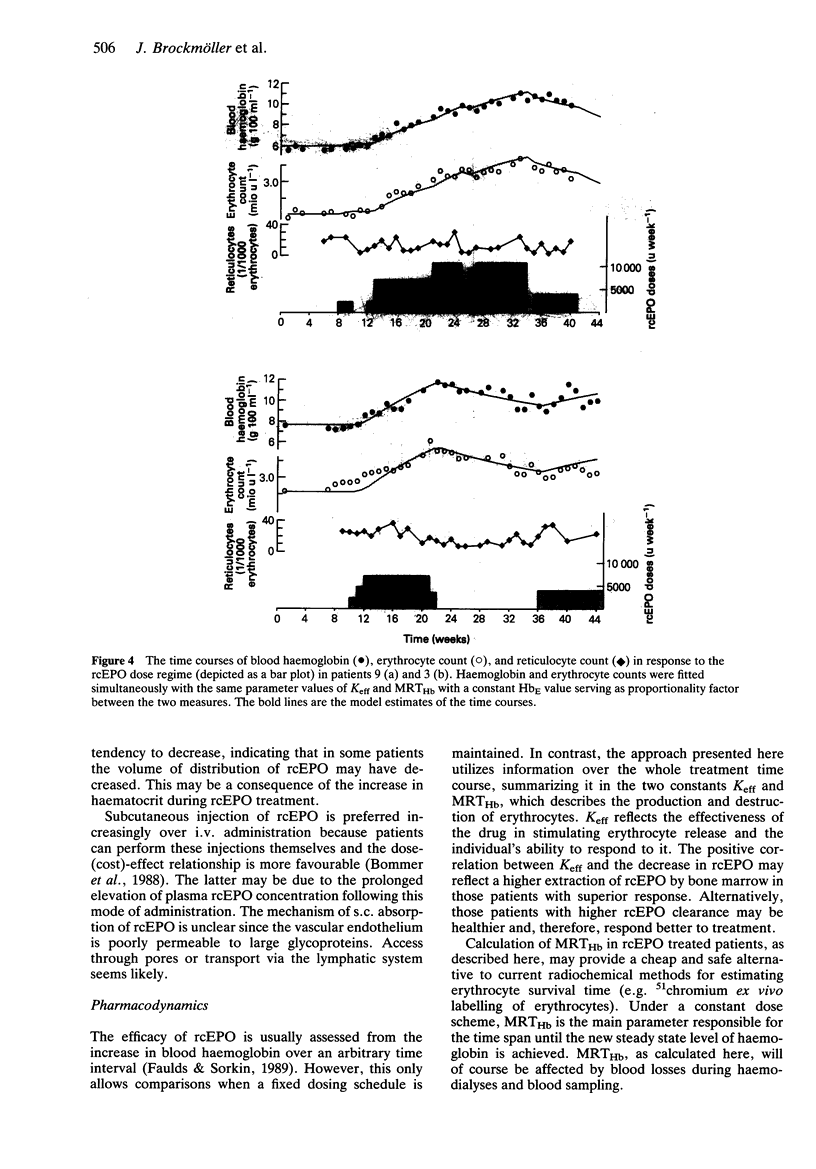
1. The pharmacokinetics of and therapeutic response to recombinant human erythropoietin (rcEPO) were studied in 12 patients under chronic haemodialysis on a thrice weekly intravenous rcEPO treatment scheme. The kinetics of rcEPO were also assessed after a subcutaneous injection during the initial period and during maintenance treatment. RcEPO was measured in plasma by radioimmunoassay. 2. After the first i.v. dose plasma erythropoietin concentrations were best described by a monoexponential disposition function with a mean (+/- s.d.) elimination half-life of 5.4 +/- 1.7 h. The volume of distribution was 70 +/- 5.2 ml kg-1 and the clearance was 10.1 +/- 3.5 ml h-1 kg-1 (n = 12). 3. After 3 months of continuous therapy, the plasma half-life of rcEPO decreased by 15% (P < 0.05, mean half-life during steady state: 4.6 +/- 2.8 h), while mean clearance and volume of distribution remained constant. 4. After the first s.c. injection the mean (+/- s.d.) absorption time was 22 +/- 11 h and systemic availability was 44 +/- 7%. 5. Changes in haemoglobin concentrations were described by a linear additive dose-response model, defined by an efficacy constant (Keff) and the mean erythrocyte lifetime (MRTHb). The sample mean (+/- s.d.) Keff was 0.043 +/- 0.017 g dl-1 Hb per 1000 units rcEPO and MRTHb was 10.02 +/- 1.75 weeks. The net effect of rcEPO treatment was described by the area under the unit-dose-response curve (AUEC) with a mean (+/- s.d.) value of 0.45 +/- 0.23 g dl-1 weeks. 6. RcEPO clearance showed a significant positive correlation (r2 = 0.41) with the effectiveness of rcEPO therapy, as measured by the parameters Keff or AUEC.
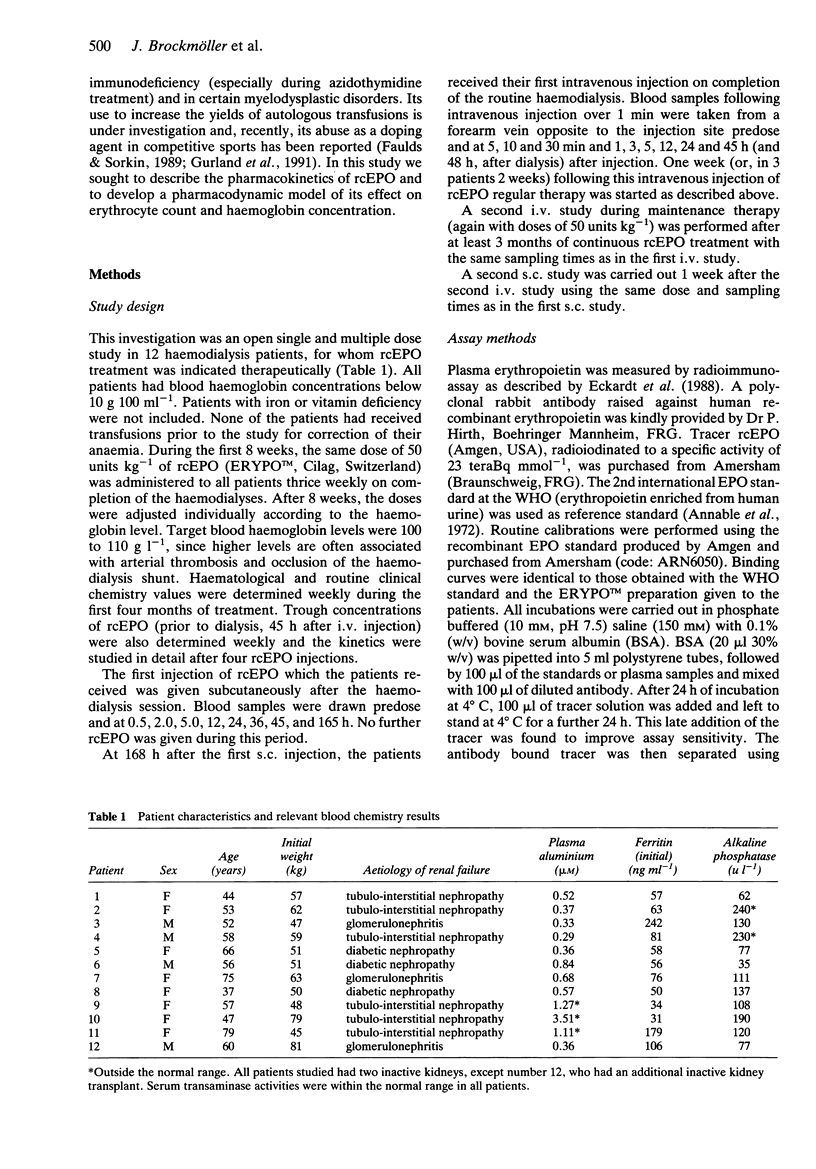
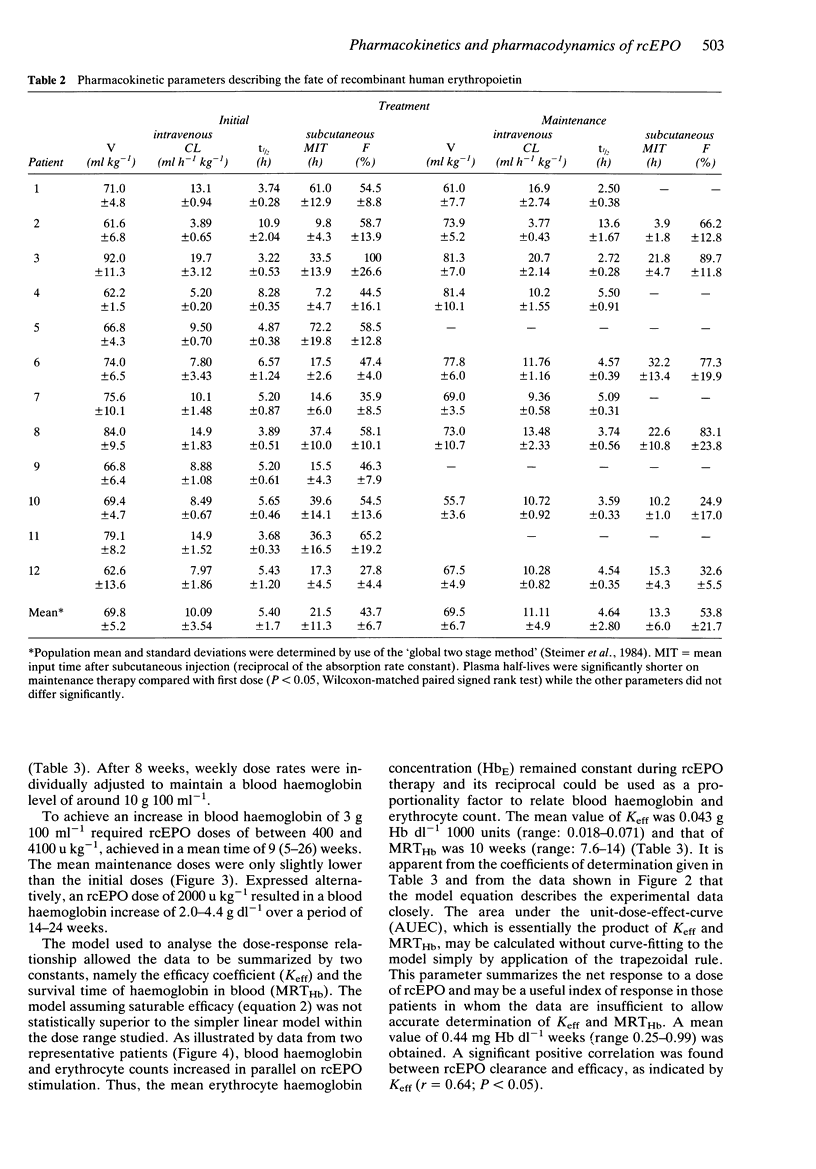
Full text
PDF

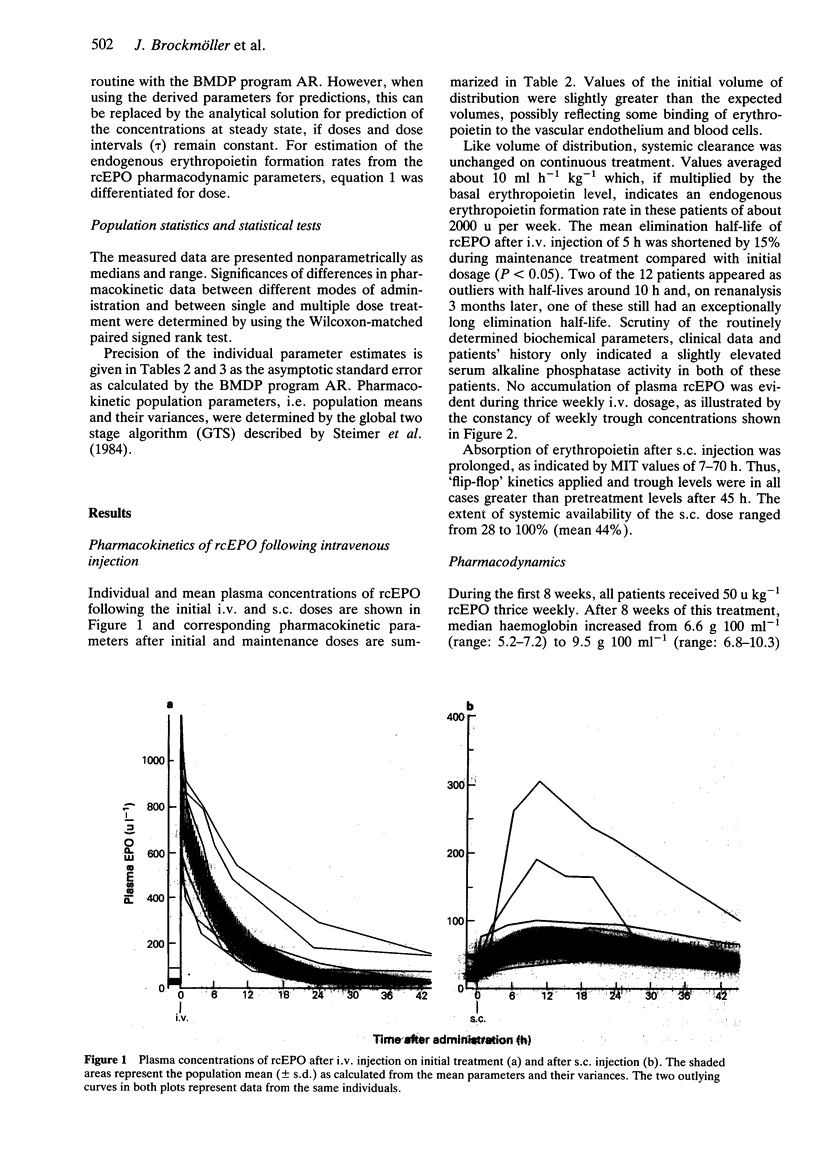


Selected References
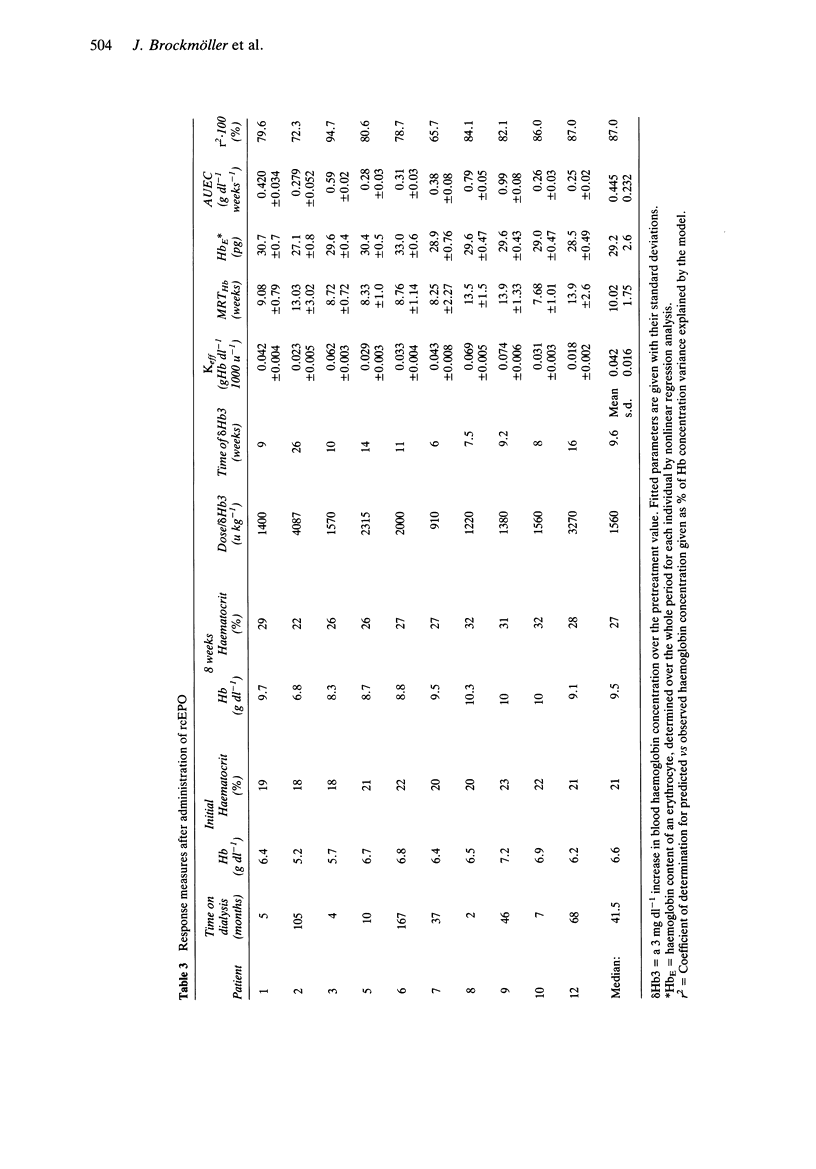
These references are in PubMed. This may not be the complete list of references from this article.
- Annable L., Cotes P. M., Mussett M. V. The second international reference preparation of erythropoietin, human, urinary, for bioassay. Bull World Health Organ. 1972;47(1):99–112. [PMC free article] [PubMed] [Google Scholar]
- Bommer J., Ritz E., Weinreich T., Bommer G., Ziegler T. Subcutaneous erythropoietin. Lancet. 1988 Aug 13;2(8607):406–406. doi: 10.1016/s0140-6736(88)92886-3. [DOI] [PubMed] [Google Scholar]
- Browne J. K., Cohen A. M., Egrie J. C., Lai P. H., Lin F. K., Strickland T., Watson E., Stebbing N. Erythropoietin: gene cloning, protein structure, and biological properties. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):693–702. doi: 10.1101/sqb.1986.051.01.082. [DOI] [PubMed] [Google Scholar]
- Cotes P. M., Pippard M. J., Reid C. D., Winearls C. G., Oliver D. O., Royston J. P. Characterization of the anaemia of chronic renal failure and the mode of its correction by a preparation of human erythropoietin (r-HuEPO). An investigation of the pharmacokinetics of intravenous erythropoietin and its effects on erythrokinetics. Q J Med. 1989 Feb;70(262):113–137. [PubMed] [Google Scholar]
- Cutler D. J. Numerical deconvolution by least squares: use of prescribed input functions. J Pharmacokinet Biopharm. 1978 Jun;6(3):227–241. doi: 10.1007/BF01312264. [DOI] [PubMed] [Google Scholar]
- Eckardt K. U., Kurtz A., Hirth P., Scigalla P., Wieczorek L., Bauer C. Evaluation of the stability of human erythropoietin in samples for radioimmunoassay. Klin Wochenschr. 1988 Mar 15;66(6):241–245. doi: 10.1007/BF01748163. [DOI] [PubMed] [Google Scholar]
- Faulds D., Sorkin E. M. Epoetin (recombinant human erythropoietin). A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in anaemia and the stimulation of erythropoiesis. Drugs. 1989 Dec;38(6):863–899. doi: 10.2165/00003495-198938060-00004. [DOI] [PubMed] [Google Scholar]
- Fisher J. W. Pharmacologic modulation of erythropoietin production. Annu Rev Pharmacol Toxicol. 1988;28:101–122. doi: 10.1146/annurev.pa.28.040188.000533. [DOI] [PubMed] [Google Scholar]
- Flaharty K. K., Caro J., Erslev A., Whalen J. J., Morris E. M., Bjornsson T. D., Vlasses P. H. Pharmacokinetics and erythropoietic response to human recombinant erythropoietin in healthy men. Clin Pharmacol Ther. 1990 May;47(5):557–564. doi: 10.1038/clpt.1990.76. [DOI] [PubMed] [Google Scholar]
- Fukuda M. N., Sasaki H., Lopez L., Fukuda M. Survival of recombinant erythropoietin in the circulation: the role of carbohydrates. Blood. 1989 Jan;73(1):84–89. [PubMed] [Google Scholar]
- Gribben J. G., Devereux S., Thomas N. S., Keim M., Jones H. M., Goldstone A. H., Linch D. C. Development of antibodies to unprotected glycosylation sites on recombinant human GM-CSF. Lancet. 1990 Feb 24;335(8687):434–437. doi: 10.1016/0140-6736(90)90665-r. [DOI] [PubMed] [Google Scholar]
- Holford N. H., Sheiner L. B. Kinetics of pharmacologic response. Pharmacol Ther. 1982;16(2):143–166. doi: 10.1016/0163-7258(82)90051-1. [DOI] [PubMed] [Google Scholar]
- Hughes R. T., Cotes P. M., Oliver D. O., Pippard M. J., Royston P., Stevens J. M., Strong C. A., Tam R. C., Winearls C. G. Correction of the anaemia of chronic renal failure with erythropoietin: pharmacokinetic studies in patients on haemodialysis and CAPD. Contrib Nephrol. 1989;76:122–130. doi: 10.1159/000417888. [DOI] [PubMed] [Google Scholar]
- Imbimbo B. P., Imbimbo E., Daniotti S., Verotta D., Bassotti G. A new criterion for selection of pharmacokinetic multiexponential equations. J Pharm Sci. 1988 Sep;77(9):784–789. doi: 10.1002/jps.2600770914. [DOI] [PubMed] [Google Scholar]
- Kampf D., Kahl A., Passlick J., Pustelnik A., Eckardt K. U., Ehmer B., Jacobs C., Baumelou A., Grabensee B., Gahl G. M. Single-dose kinetics of recombinant human erythropoietin after intravenous, subcutaneous and intraperitoneal administration. Preliminary results. Contrib Nephrol. 1989;76:106–111. doi: 10.1159/000417886. [DOI] [PubMed] [Google Scholar]
- Lim V. S., DeGowin R. L., Zavala D., Kirchner P. T., Abels R., Perry P., Fangman J. Recombinant human erythropoietin treatment in pre-dialysis patients. A double-blind placebo-controlled trial. Ann Intern Med. 1989 Jan 15;110(2):108–114. doi: 10.7326/0003-4819-110-2-108. [DOI] [PubMed] [Google Scholar]
- Lui S. F., Chung W. W., Leung C. B., Chan K., Lai K. N. Pharmacokinetics and pharmacodynamics of subcutaneous and intraperitoneal administration of recombinant human erythropoietin in patients on continuous ambulatory peritoneal dialysis. Clin Nephrol. 1990 Jan;33(1):47–51. [PubMed] [Google Scholar]
- Macdougall I. C., Roberts D. E., Coles G. A., Williams J. D. Clinical pharmacokinetics of epoetin (recombinant human erythropoietin). Clin Pharmacokinet. 1991 Feb;20(2):99–113. doi: 10.2165/00003088-199120020-00002. [DOI] [PubMed] [Google Scholar]
- Macdougall I. C., Roberts D. E., Neubert P., Dharmasena A. D., Coles G. A., Williams J. D. Pharmacokinetics of intravenous, intraperitoneal, and subcutaneous recombinant erythropoietin in patients on CAPD. A rationale for treatment. Contrib Nephrol. 1989;76:112–121. doi: 10.1159/000417887. [DOI] [PubMed] [Google Scholar]
- Macdougall I. C., Roberts D. E., Neubert P., Dharmasena A. D., Coles G. A., Williams J. D. Pharmacokinetics of recombinant human erythropoietin in patients on continuous ambulatory peritoneal dialysis. Lancet. 1989 Feb 25;1(8635):425–427. doi: 10.1016/s0140-6736(89)90014-7. [DOI] [PubMed] [Google Scholar]
- McMahon F. G., Vargas R., Ryan M., Jain A. K., Abels R. I., Perry B., Smith I. L. Pharmacokinetics and effects of recombinant human erythropoietin after intravenous and subcutaneous injections in healthy volunteers. Blood. 1990 Nov 1;76(9):1718–1722. [PubMed] [Google Scholar]
- Neumayer H. H., Brockmöller J., Fritschka E., Roots I., Scigalla P., Wattenberg M. Pharmacokinetics of recombinant human erythropoietin after SC administration and in long-term IV treatment in patients on maintenance hemodialysis. Contrib Nephrol. 1989;76:131–142. doi: 10.1159/000417889. [DOI] [PubMed] [Google Scholar]
- Spivak J. L., Hogans B. B. The in vivo metabolism of recombinant human erythropoietin in the rat. Blood. 1989 Jan;73(1):90–99. [PubMed] [Google Scholar]
- Steimer J. L., Mallet A., Golmard J. L., Boisvieux J. F. Alternative approaches to estimation of population pharmacokinetic parameters: comparison with the nonlinear mixed-effect model. Drug Metab Rev. 1984;15(1-2):265–292. doi: 10.3109/03602538409015066. [DOI] [PubMed] [Google Scholar]
- Stone W. J., Graber S. E., Krantz S. B., Dessypris E. N., O'Neil V. L., Olsen N. J., Pincus T. P. Treatment of the anemia of predialysis patients with recombinant human erythropoietin: a randomized, placebo-controlled trial. Am J Med Sci. 1988 Sep;296(3):171–179. doi: 10.1097/00000441-198809000-00005. [DOI] [PubMed] [Google Scholar]
- Uehlinger D. E., Gotch F. A., Sheiner L. B. A pharmacodynamic model of erythropoietin therapy for uremic anemia. Clin Pharmacol Ther. 1992 Jan;51(1):76–89. doi: 10.1038/clpt.1992.10. [DOI] [PubMed] [Google Scholar]
- Urabe A., Takaku F., Mizoguchi H., Kubo K., Ota K., Shimizu N., Tanaka K., Mimura N., Nihei H., Koshikawa S. Effect of recombinant human erythropoietin on the anemia of chronic renal failure. Int J Cell Cloning. 1988 May;6(3):179–191. doi: 10.1002/stem.5530060304. [DOI] [PubMed] [Google Scholar]
- Verotta D. Comments on two recent deconvolution methods. J Pharmacokinet Biopharm. 1990 Oct;18(5):483–499. doi: 10.1007/BF01061706. [DOI] [PubMed] [Google Scholar]
- van Rossum J. M., de Bie J. E., van Lingen G., Teeuwen H. W. Pharmacokinetics from a dynamical systems point of view. J Pharmacokinet Biopharm. 1989 Jun;17(3):365–400. doi: 10.1007/BF01061902. [DOI] [PubMed] [Google Scholar]


