Abstract
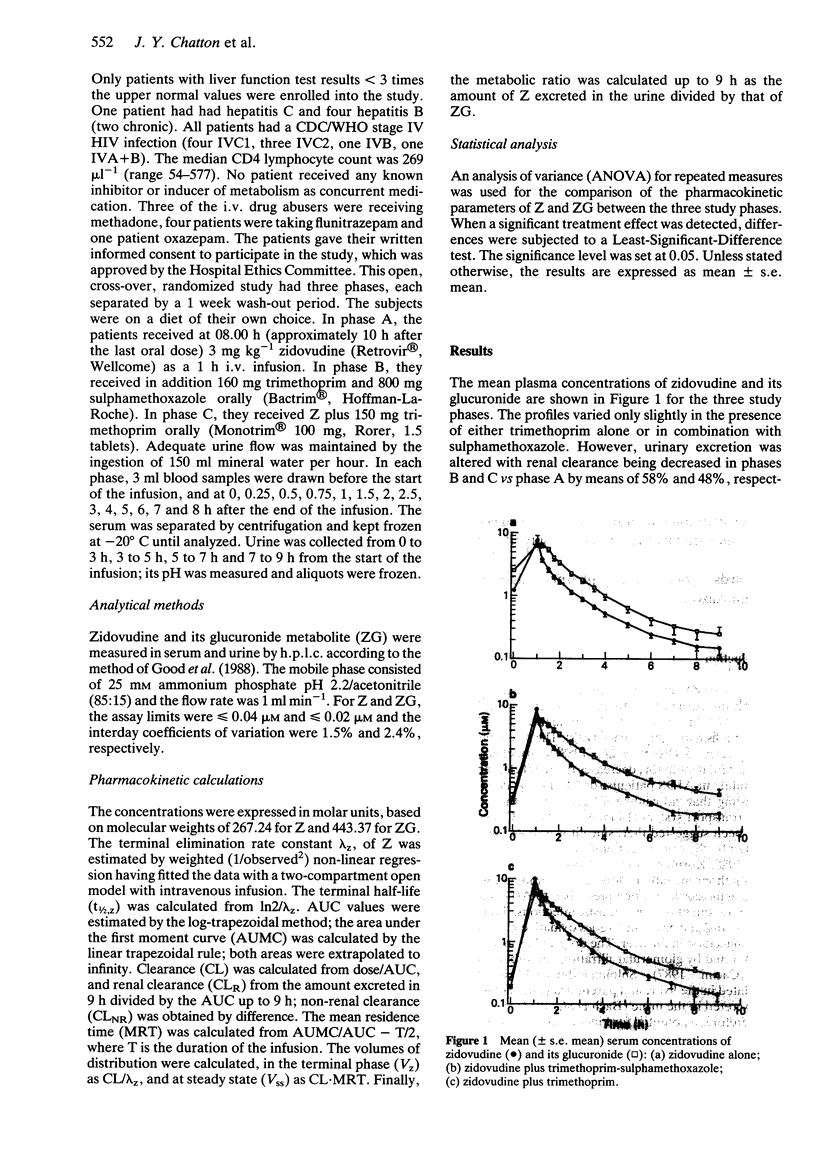
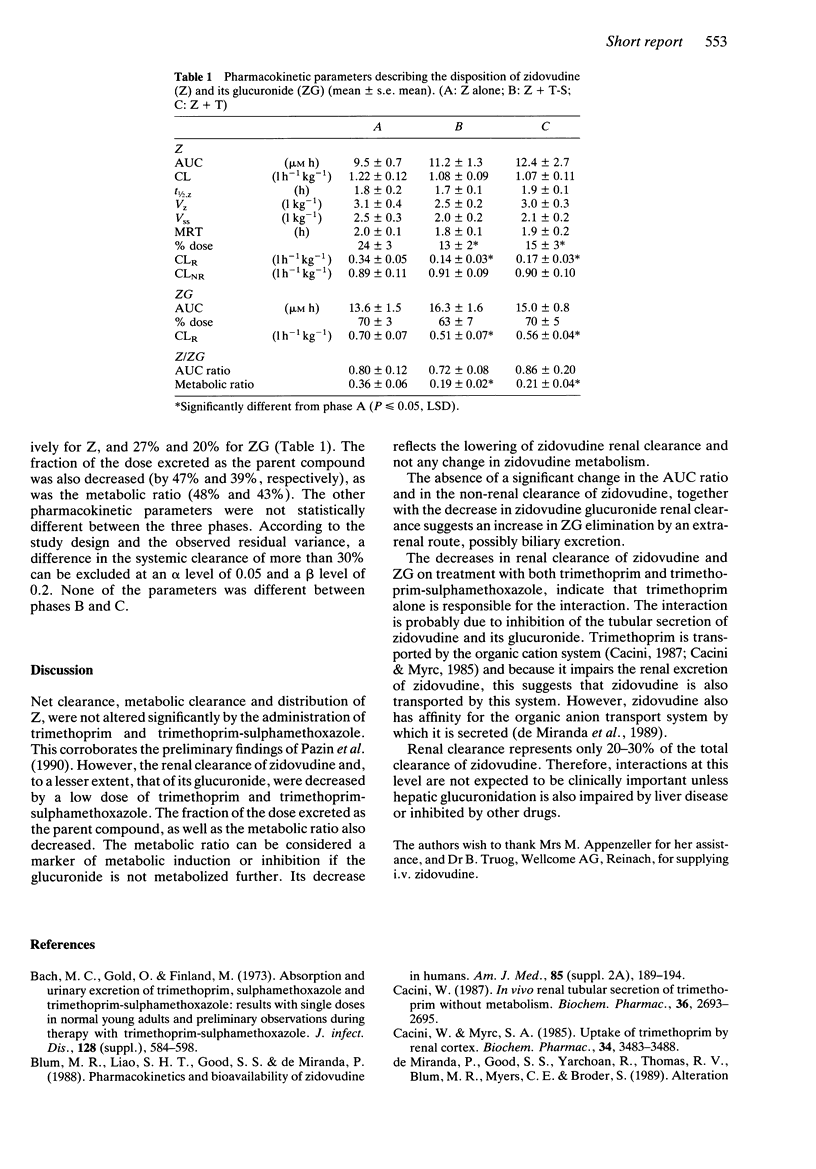
Trimethoprim and trimethoprim-sulphamethoxazole (co-trimoxazole) are often prescribed in HIV patients treated with zidovudine. The pharmacokinetics of zidovudine, after a dose of 3 mg kg-1 by constant rate intravenous infusion over 1 h were evaluated in nine HIV patients in an open, randomized, three-phase crossover study, without and with trimethoprim (150 mg) and trimethoprim-sulphamethoxazole (160 and 800 mg). The metabolic clearance of zidovudine was not significantly influenced by trimethoprim-sulphamethoxazole and trimethoprim. However, the renal clearance of zidovudine was decreased by 58 and 48%, respectively, and that of its glucuronide by 27 and 20% (P < 0.05). The fraction of the dose excreted as the parent compound fell by 47 and 39% and the metabolic ratio by 48 and 43% (P < 0.05). This kinetic drug interaction, apparently due solely to trimethoprim, may only be clinically important when hepatic glucuronidation is also impaired by liver disease or inhibited by other drugs.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. C., Gold O., Finland M. Absorption and urinary execretion of trimethoprim, sulfamethoxazole, and trimethoprim-sulfamethoxazole: results with single doses in normal young adults and preliminary observations during therapy with trimethoprim-sulfamethoxazole. J Infect Dis. 1973 Nov;128(Suppl):584–p. doi: 10.1093/infdis/128.supplement_3.s584. [DOI] [PubMed] [Google Scholar]
- Blum M. R., Liao S. H., Good S. S., de Miranda P. Pharmacokinetics and bioavailability of zidovudine in humans. Am J Med. 1988 Aug 29;85(2A):189–194. [PubMed] [Google Scholar]
- Cacini W. In vivo renal tubular secretion of trimethoprim without metabolism. Biochem Pharmacol. 1987 Aug 15;36(16):2693–2695. doi: 10.1016/0006-2952(87)90556-9. [DOI] [PubMed] [Google Scholar]
- Cacini W., Myre S. A. Uptake of trimethoprim by renal cortex. Biochem Pharmacol. 1985 Oct 1;34(19):3483–3488. doi: 10.1016/0006-2952(85)90721-x. [DOI] [PubMed] [Google Scholar]
- Good S. S., Reynolds D. J., de Miranda P. Simultaneous quantification of zidovudine and its glucuronide in serum by high-performance liquid chromatography. J Chromatogr. 1988 Sep 23;431(1):123–133. doi: 10.1016/s0378-4347(00)83075-3. [DOI] [PubMed] [Google Scholar]
- Henry K., Chinnock B. J., Quinn R. P., Fletcher C. V., de Miranda P., Balfour H. H., Jr Concurrent zidovudine levels in semen and serum determined by radioimmunoassay in patients with AIDS or AIDS-related complex. JAMA. 1988 May 27;259(20):3023–3026. [PubMed] [Google Scholar]
- Kornhauser D. M., Petty B. G., Hendrix C. W., Woods A. S., Nerhood L. J., Bartlett J. G., Lietman P. S. Probenecid and zidovudine metabolism. Lancet. 1989 Aug 26;2(8661):473–475. doi: 10.1016/s0140-6736(89)92087-4. [DOI] [PubMed] [Google Scholar]
- Mays D. C., Dixon K. F., Balboa A., Pawluk L. J., Bauer M. R., Nawoot S., Gerber N. A nonprimate animal model applicable to zidovudine pharmacokinetics in humans: inhibition of glucuronidation and renal excretion of zidovudine by probenecid in rats. J Pharmacol Exp Ther. 1991 Dec;259(3):1261–1270. [PubMed] [Google Scholar]
- Yarchoan R., Mitsuya H., Myers C. E., Broder S. Clinical pharmacology of 3'-azido-2',3'-dideoxythymidine (zidovudine) and related dideoxynucleosides. N Engl J Med. 1989 Sep 14;321(11):726–738. doi: 10.1056/NEJM198909143211106. [DOI] [PubMed] [Google Scholar]
- de Miranda P., Good S. S., Yarchoan R., Thomas R. V., Blum M. R., Myers C. E., Broder S. Alteration of zidovudine pharmacokinetics by probenecid in patients with AIDS or AIDS-related complex. Clin Pharmacol Ther. 1989 Nov;46(5):494–500. doi: 10.1038/clpt.1989.176. [DOI] [PubMed] [Google Scholar]


