Abstract
1. The responsiveness of human and bovine bronchi was examined by comparing the magnitude of responses to agonists applied to the adventitial (outside) and to the luminal (inside) surfaces. The development of smooth muscle tone was measured as both an increase in pressure in isovolumic bronchial segments and narrowing in perfused segments. 2. In both closed and perfused human segments, ACh added to the adventitial surface produced highly homogeneous responses while responses to ACh added to the luminal side were extremely variable. 3. Histological examination of the human segments showed that they possessed variable degrees of pre-existing epithelial loss from the mucosal circumference, ranging from 0 to 82%. 4. Denuded human isovolumic segments (exhibiting > 30% epithelial loss) were 40 fold more sensitive to ACh inside than intact segments (< 30%). Denuded segments were also equally responsive to KCl inside or outside while KCl inside produced a contraction that was 50% of that to KCl outside in intact segments. 5. A strong and highly significant relationship was determined between the proportion of epithelial loss and both the responsiveness and rate of contraction of human segments to ACh and KCl introduced onto the lumen. No relationship was observed between epithelial denudation and responsiveness or contractility to agonists added to the adventitial surface. 6. Mechanical denudation of the epithelium from bovine segments had no effect on responsiveness to ACh or KCl added to the outside while significantly augmenting the sensitivity to ACh (9 fold) and reactivity to KCl introduced into the lumen to the extent that it became the same as outside.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

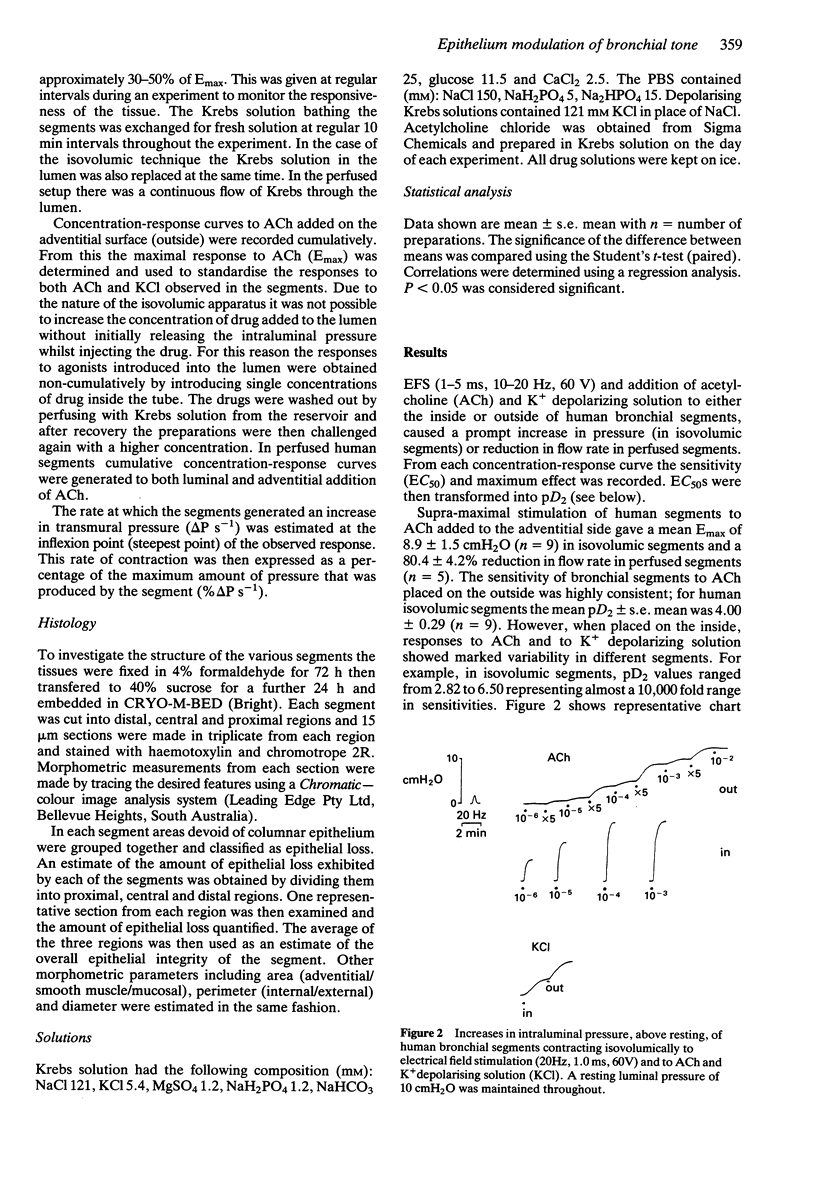

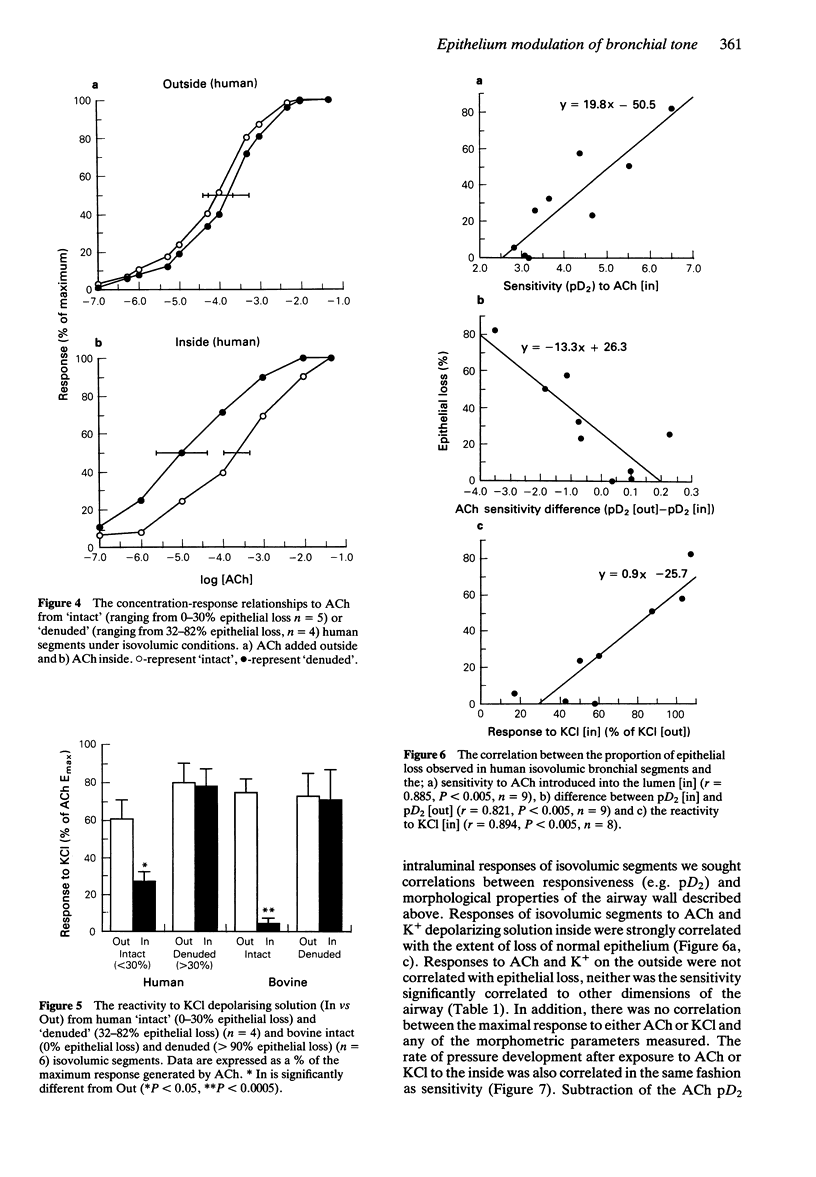
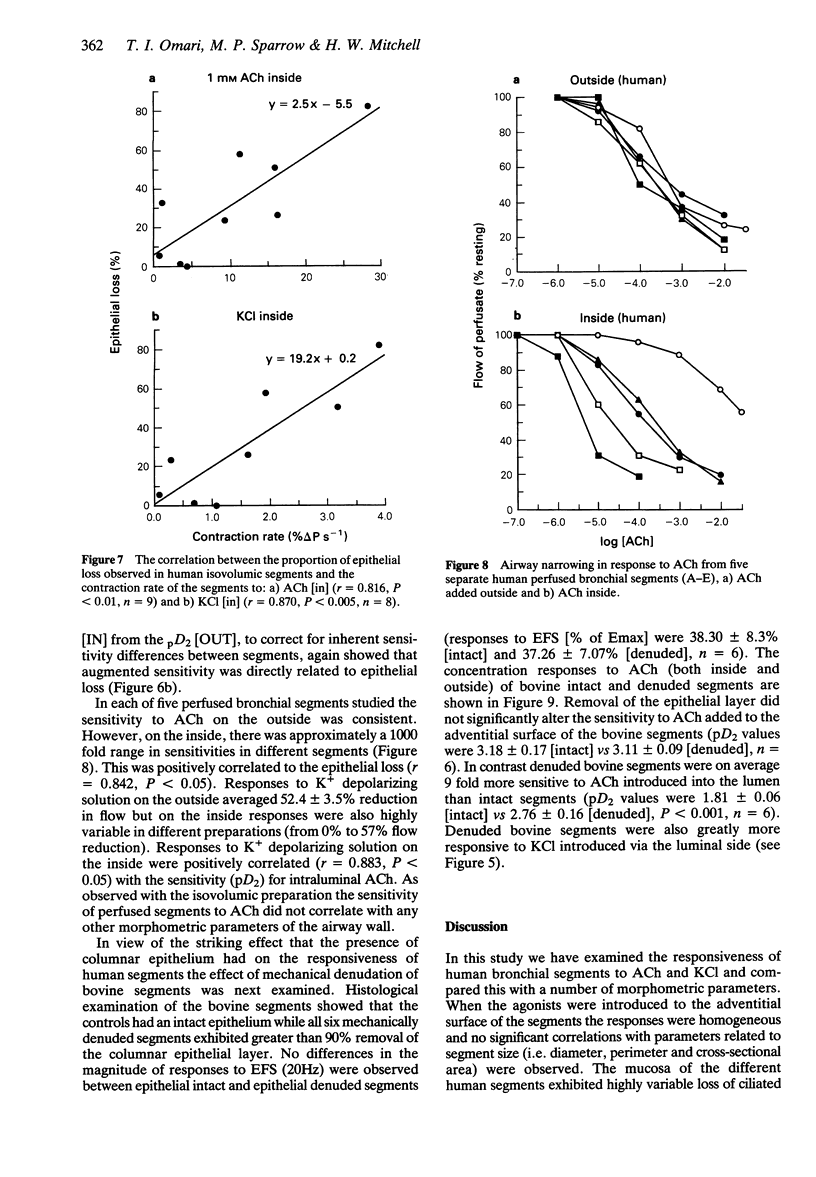


Images in this article
Selected References
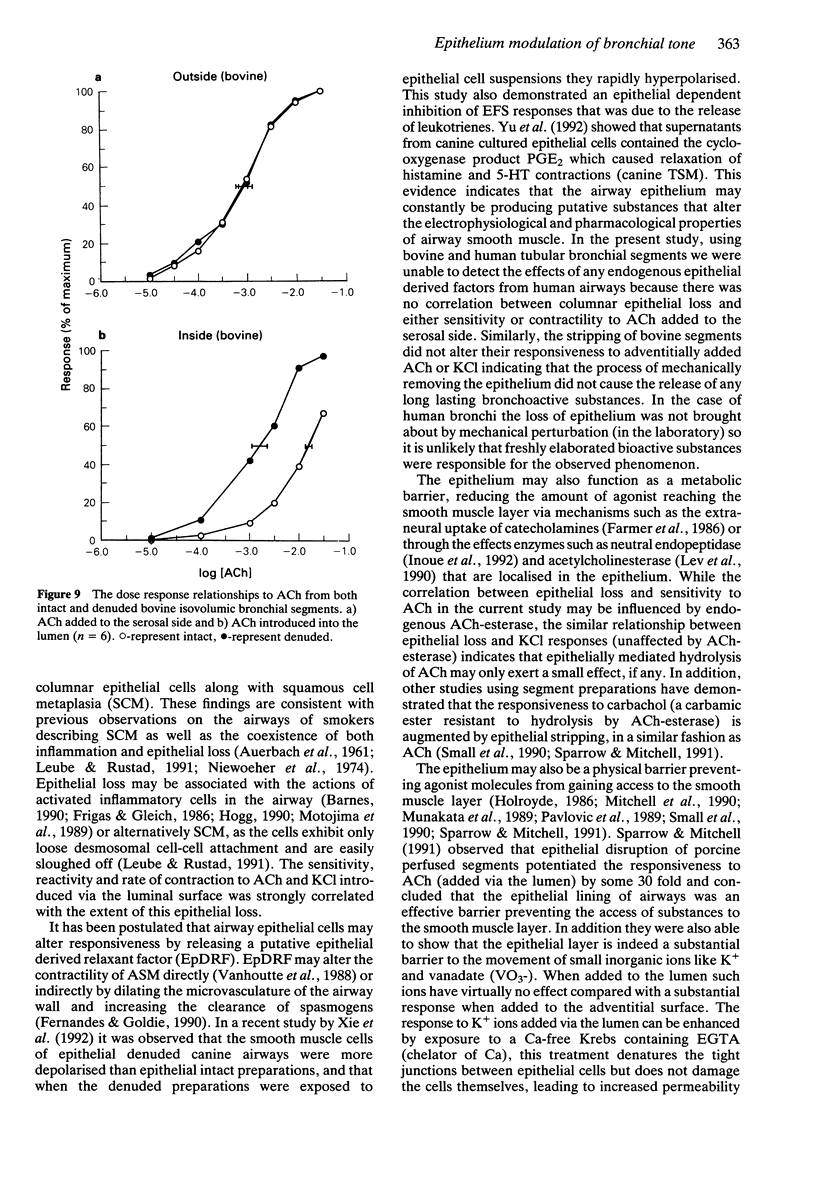
These references are in PubMed. This may not be the complete list of references from this article.
- AUERBACH O., STOUT A. P., HAMMOND E. C., GARFINKEL L. Changes in bronchial epithelium in relation to cigarette smoking and in relation to lung cancer. N Engl J Med. 1961 Aug 10;265:253–267. doi: 10.1056/NEJM196108102650601. [DOI] [PubMed] [Google Scholar]
- Armour C. L., Lazar N. M., Schellenberg R. R., Taylor S. M., Chan N., Hogg J. C., Paré P. D. A comparison of in vivo and in vitro human airway reactivity to histamine. Am Rev Respir Dis. 1984 Jun;129(6):907–910. doi: 10.1164/arrd.1984.129.6.907. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Cuss F. M., Palmer J. B. The effect of airway epithelium on smooth muscle contractility in bovine trachea. Br J Pharmacol. 1985 Nov;86(3):685–691. doi: 10.1111/j.1476-5381.1985.tb08946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J. Reactive oxygen species and airway inflammation. Free Radic Biol Med. 1990;9(3):235–243. doi: 10.1016/0891-5849(90)90034-g. [DOI] [PubMed] [Google Scholar]
- Farmer S. G., Fedan J. S., Hay D. W., Raeburn D. The effects of epithelium removal on the sensitivity of guinea-pig isolated trachealis to bronchodilator drugs. Br J Pharmacol. 1986 Oct;89(2):407–414. doi: 10.1111/j.1476-5381.1986.tb10274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes L. B., Goldie R. G. Pharmacological evaluation of a guinea-pig tracheal epithelium-derived inhibitory factor (EpDIF). Br J Pharmacol. 1990 Jul;100(3):614–618. doi: 10.1111/j.1476-5381.1990.tb15855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigas E., Gleich G. J. The eosinophil and the pathophysiology of asthma. J Allergy Clin Immunol. 1986 Apr;77(4):527–537. doi: 10.1016/0091-6749(86)90341-6. [DOI] [PubMed] [Google Scholar]
- Goldie R. G., Fernandes L. B., Farmer S. G., Hay D. W. Airway epithelium-derived inhibitory factor. Trends Pharmacol Sci. 1990 Feb;11(2):67–70. doi: 10.1016/0165-6147(90)90320-8. [DOI] [PubMed] [Google Scholar]
- Goldie R. G., Papadimitriou J. M., Paterson J. W., Rigby P. J., Self H. M., Spina D. Influence of the epithelium on responsiveness of guinea-pig isolated trachea to contractile and relaxant agonists. Br J Pharmacol. 1986 Jan;87(1):5–14. doi: 10.1111/j.1476-5381.1986.tb10150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R. E., Solano D., Kleinerman J. Tight junction alterations of respiratory epithelium following long-term NO2 exposure and recovery. Exp Lung Res. 1986;11(3):179–193. doi: 10.3109/01902148609064295. [DOI] [PubMed] [Google Scholar]
- Hogg J. C. Mucosal permeability and smooth muscle function in asthma. Med Clin North Am. 1990 May;74(3):731–740. doi: 10.1016/s0025-7125(16)30548-x. [DOI] [PubMed] [Google Scholar]
- Holroyde M. C. The influence of epithelium on the responsiveness of guinea-pig isolated trachea. Br J Pharmacol. 1986 Mar;87(3):501–507. doi: 10.1111/j.1476-5381.1986.tb10192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Sakai Y., Homma I. An ubiquitous modulating function of rabbit tracheal epithelium: degradation of tachykinins. Br J Pharmacol. 1992 Feb;105(2):393–399. doi: 10.1111/j.1476-5381.1992.tb14264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery P. K., Wardlaw A. J., Nelson F. C., Collins J. V., Kay A. B. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989 Dec;140(6):1745–1753. doi: 10.1164/ajrccm/140.6.1745. [DOI] [PubMed] [Google Scholar]
- Kennedy S. M., Elwood R. K., Wiggs B. J., Paré P. D., Hogg J. C. Increased airway mucosal permeability of smokers. Relationship to airway reactivity. Am Rev Respir Dis. 1984 Jan;129(1):143–148. doi: 10.1164/arrd.1984.129.1.143. [DOI] [PubMed] [Google Scholar]
- Köhn H., König B., Klech H., Pohl W., Mostbeck A. Urine excretion of inhaled technetium-99m-DTPA: an alternative method to assess lung epithelial transport. J Nucl Med. 1990 Apr;31(4):441–449. [PubMed] [Google Scholar]
- Leube R. E., Rustad T. J. Squamous cell metaplasia in the human lung: molecular characteristics of epithelial stratification. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;61(4):227–253. doi: 10.1007/BF02890425. [DOI] [PubMed] [Google Scholar]
- Lev A., Christensen G. C., Zhang R. A., Kelsen S. G. Epithelial effects on tracheal smooth muscle tone: influence of muscarinic antagonists. Am J Physiol. 1990 Feb;258(2 Pt 1):L52–L56. doi: 10.1152/ajplung.1990.258.2.L52. [DOI] [PubMed] [Google Scholar]
- Lundblad K. A., Persson C. G. The epithelium and the pharmacology of guinea-pig tracheal tone in vitro. Br J Pharmacol. 1988 Apr;93(4):909–917. doi: 10.1111/j.1476-5381.1988.tb11479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H., Setoguti T. Freeze-fracture replica studies of tight junctions in normal human bronchial epithelium. Acta Anat (Basel) 1989;134(3):219–226. doi: 10.1159/000146690. [DOI] [PubMed] [Google Scholar]
- Mitchell H. W., Willet K. E., Sparrow M. P. Perfused bronchial segment and bronchial strip: narrowing vs. isometric force by mediators. J Appl Physiol (1985) 1989 Jun;66(6):2704–2709. doi: 10.1152/jappl.1989.66.6.2704. [DOI] [PubMed] [Google Scholar]
- Mitchell H. W., Willet K. E., Sparrow M. P. The role of epithelium in the responsiveness of the bronchi to stimuli. Agents Actions Suppl. 1990;31:275–278. doi: 10.1007/978-3-0348-7379-6_37. [DOI] [PubMed] [Google Scholar]
- Motojima S., Frigas E., Loegering D. A., Gleich G. J. Toxicity of eosinophil cationic proteins for guinea pig tracheal epithelium in vitro. Am Rev Respir Dis. 1989 Mar;139(3):801–805. doi: 10.1164/ajrccm/139.3.801. [DOI] [PubMed] [Google Scholar]
- Munakata M., Huang I., Mitzner W., Menkes H. Protective role of epithelium in the guinea pig airway. J Appl Physiol (1985) 1989 Apr;66(4):1547–1552. doi: 10.1152/jappl.1989.66.4.1547. [DOI] [PubMed] [Google Scholar]
- Niewoehner D. E., Kleinerman J., Rice D. B. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974 Oct 10;291(15):755–758. doi: 10.1056/NEJM197410102911503. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Ikeda H., Fukuda T., Suzuki S., Okubo T. Acute exposure to cigarette smoke induces airway hyperresponsiveness without airway inflammation in guinea pigs. Dose-response characteristics. Am Rev Respir Dis. 1990 Jul;142(1):177–183. doi: 10.1164/ajrccm/142.1.177. [DOI] [PubMed] [Google Scholar]
- O'Byrne P. M., Dolovich M., Dirks R., Roberts R. S., Newhouse M. T. Lung epithelial permeability: relation to nonspecific airway responsiveness. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jul;57(1):77–84. doi: 10.1152/jappl.1984.57.1.77. [DOI] [PubMed] [Google Scholar]
- Omari T. I., Sparrow M. P. Epithelial disruption by proteases augments the responsiveness of porcine bronchial segments. Clin Exp Pharmacol Physiol. 1992 Nov;19(11):785–794. doi: 10.1111/j.1440-1681.1992.tb00416.x. [DOI] [PubMed] [Google Scholar]
- Pavlovic D., Fournier M., Aubier M., Pariente R. Epithelial vs. serosal stimulation of tracheal muscle: role of epithelium. J Appl Physiol (1985) 1989 Dec;67(6):2522–2526. doi: 10.1152/jappl.1989.67.6.2522. [DOI] [PubMed] [Google Scholar]
- Roberts J. A., Raeburn D., Rodger I. W., Thomson N. C. Comparison of in vivo airway responsiveness and in vitro smooth muscle sensitivity to methacholine in man. Thorax. 1984 Nov;39(11):837–843. doi: 10.1136/thx.39.11.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simani A. S., Inoue S., Hogg J. C. Penetration of the respiratory epithelium of guinea pigs following exposure to cigarette smoke. Lab Invest. 1974 Jul;31(1):75–81. [PubMed] [Google Scholar]
- Small R. C., Good D. M., Dixon J. S., Kennedy I. The effects of epithelium removal on the actions of cholinomimetic drugs in opened segments and perfused tubular preparations of guinea-pig trachea. Br J Pharmacol. 1990 Jul;100(3):516–522. doi: 10.1111/j.1476-5381.1990.tb15839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow M. P., Mitchell H. W. Modulation by the epithelium of the extent of bronchial narrowing produced by substances perfused through the lumen. Br J Pharmacol. 1991 May;103(1):1160–1164. doi: 10.1111/j.1476-5381.1991.tb12317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart-Smith K., Vanhoutte P. M. Airway epithelium modulates the responsiveness of porcine bronchial smooth muscle. J Appl Physiol (1985) 1988 Aug;65(2):721–727. doi: 10.1152/jappl.1988.65.2.721. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M. Epithelium-derived relaxing factor(s) and bronchial reactivity. Am Rev Respir Dis. 1988 Dec;138(6 Pt 2):S24–S30. doi: 10.1164/ajrccm/138.6_Pt_2.S24. [DOI] [PubMed] [Google Scholar]
- Vincenc K. S., Black J. L., Yan K., Armour C. L., Donnelly P. D., Woolcock A. J. Comparison of in vivo and in vitro responses to histamine in human airways. Am Rev Respir Dis. 1983 Nov;128(5):875–879. doi: 10.1164/arrd.1983.128.5.875. [DOI] [PubMed] [Google Scholar]
- Xie Z., Hakoda H., Ito Y. Airway epithelial cells regulate membrane potential, neurotransmission and muscle tone of the dog airway smooth muscle. J Physiol. 1992 Apr;449:619–639. doi: 10.1113/jphysiol.1992.sp019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. Y., Hubbard W., Spannhake E. W. Inhibition of canine tracheal smooth muscle by mediators from cultured bronchial epithelial cells. Am J Physiol. 1992 Feb;262(2 Pt 1):L229–L234. doi: 10.1152/ajplung.1992.262.2.L229. [DOI] [PubMed] [Google Scholar]



