Abstract
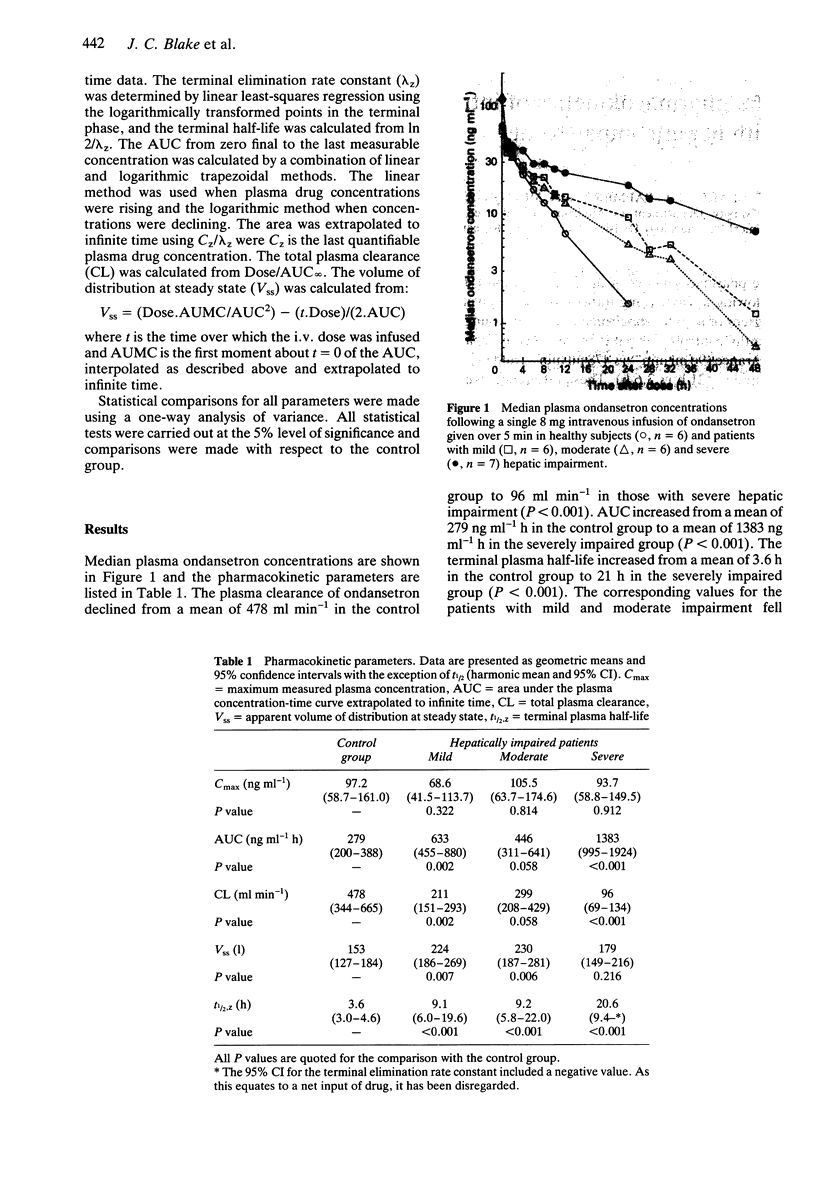
The pharmacokinetics of the 5-HT3 receptor antagonist ondansetron were investigated following a single 8 mg intravenous dose given over 5 min in 19 patients with varying degrees of hepatic impairment and in six young healthy subjects. In comparison with the healthy controls, the patients with severe hepatic impairment had a lower mean plasma clearance (96 ml min-1 vs 478 ml min-1) and increased AUC (1383 ng ml-1 h vs 279 ng ml-1 h) and t1/2 (21 h vs 3.6 h). These differences were all statistically significant (P < 0.001). The corresponding values for patients with mild or moderate hepatic impairment fell between these extremes. Vss was greater in all patient groups than the control group, but the magnitude of the change was smaller than for the other parameters and did not reflect the increasing severity of hepatic impairment. There were no significant changes in Cmax. There were no drug-related adverse events in the patients studied. It is recommended that the dosing frequency of ondansetron be limited to once daily in patients with severe hepatic impairment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colthup P. V., Felgate C. C., Palmer J. L., Scully N. L. Determination of ondansetron in plasma and its pharmacokinetics in the young and elderly. J Pharm Sci. 1991 Sep;80(9):868–871. doi: 10.1002/jps.2600800913. [DOI] [PubMed] [Google Scholar]
- Howden C. W., Birnie G. G., Brodie M. J. Drug metabolism in liver disease. Pharmacol Ther. 1989;40(3):439–474. doi: 10.1016/0163-7258(89)90088-0. [DOI] [PubMed] [Google Scholar]
- Pritchard J. F., Bryson J. C., Kernodle A. E., Benedetti T. L., Powell J. R. Age and gender effects on ondansetron pharmacokinetics: evaluation of healthy aged volunteers. Clin Pharmacol Ther. 1992 Jan;51(1):51–55. doi: 10.1038/clpt.1992.7. [DOI] [PubMed] [Google Scholar]
- Saynor D. A., Dixon C. M. The metabolism of ondansetron. Eur J Cancer Clin Oncol. 1989;25 (Suppl 1):S75–S77. [PubMed] [Google Scholar]
- Tyers M. B., Bunce K. T., Humphrey P. P. Pharmacological and anti-emetic properties of ondansetron. Eur J Cancer Clin Oncol. 1989;25 (Suppl 1):S15–S19. [PubMed] [Google Scholar]


