Abstract
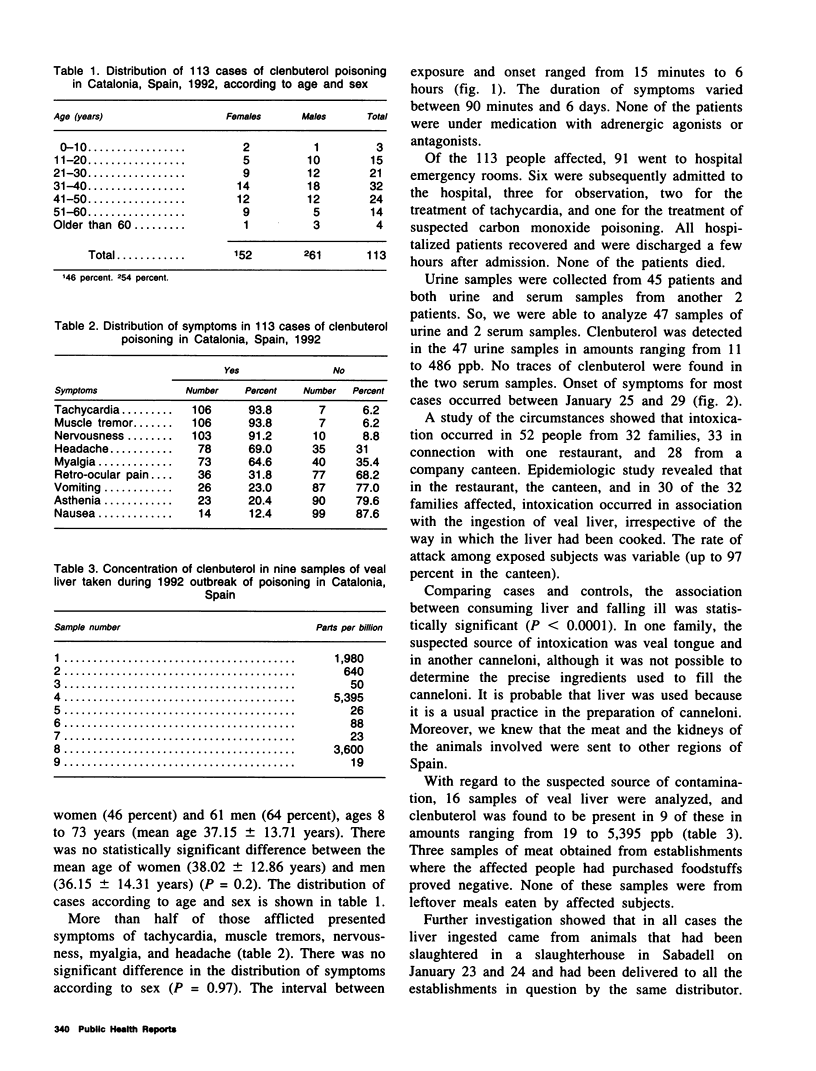
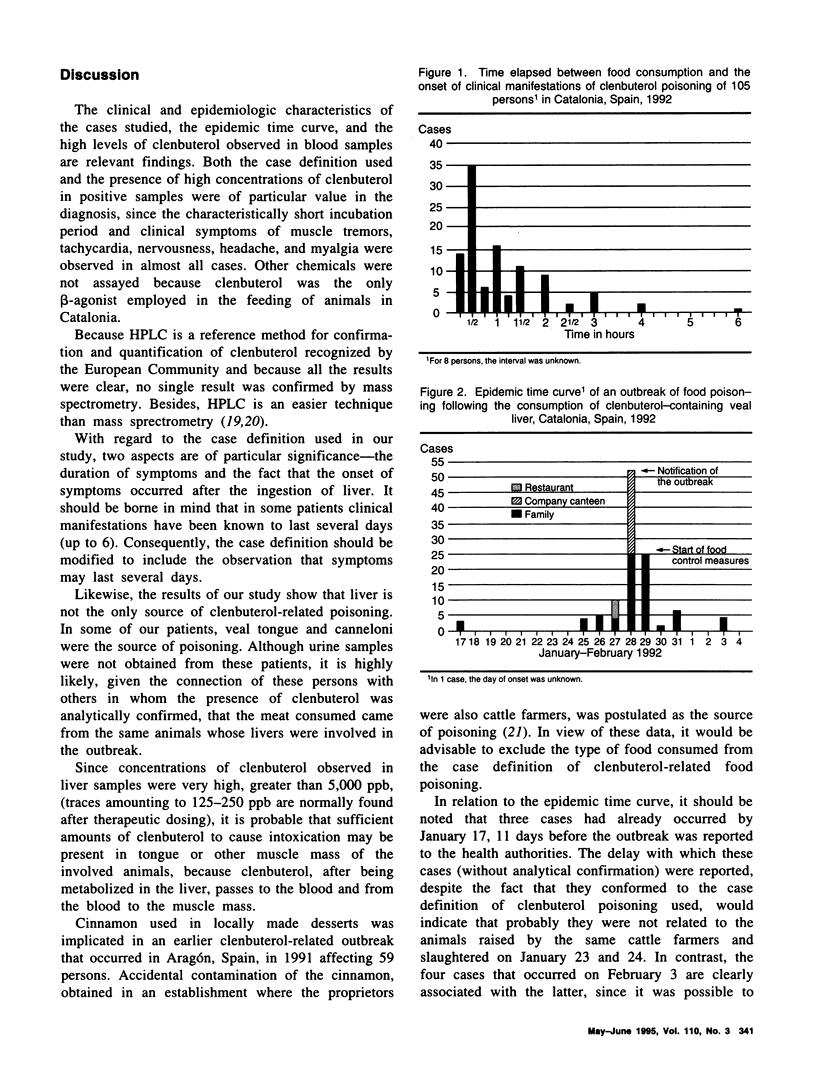
In an investigation of 113 cases of clenbuterol poisoning in Catalonia, Spain, in 1992, more than 50 percent of those affected were found to have had symptoms of nervousness, tachycardia, muscle tremors, myalgia, and headache. There was no significant difference in the distribution of symptoms according to sex (P = 0.97). The period of incubation varied between 15 minutes and 6 hours and the duration of symptoms between 90 minutes and 6 days. Clenbuterol was detected in 47 urine samples in amounts ranging from 11 to 486 parts per billion. No traces of clenbuterol were found in serum samples. Intoxication occurred in association with the ingestion of veal liver, irrespective of the way in which the liver had been cooked. The association between consuming liver and falling ill was statistically significant (P < 0.0001). In one family, the suspected source of intoxication was meat (veal tongue) and in another canneloni. None of the patients died as a result of the intoxication. The findings reinforce the need to uphold the prohibition of the use of clenbuterol in cattle farming in those countries and communities where it already exists and to contemplate a stricter regulation of its therapeutic use.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bostedt H. Zur Anwendung eines beta 2-Mimetikums (Clenbuterol) bei Graviditätsstörungen und in der Geburtshilfe des Pferdes. Tierarztl Prax. 1988;16(1):57–59. [PubMed] [Google Scholar]
- Brockway J. M., MacRae J. C., Williams P. E. Side effects of clenbuterol as a repartitioning agent. Vet Rec. 1987 Apr 18;120(16):381–383. doi: 10.1136/vr.120.16.381. [DOI] [PubMed] [Google Scholar]
- Cheung D., Timmers M. C., Zwinderman A. H., Bel E. H., Dijkman J. H., Sterk P. J. Long-term effects of a long-acting beta 2-adrenoceptor agonist, salmeterol, on airway hyperresponsiveness in patients with mild asthma. N Engl J Med. 1992 Oct 22;327(17):1198–1203. doi: 10.1056/NEJM199210223271703. [DOI] [PubMed] [Google Scholar]
- Claeys M. C., Mulvaney D. R., McCarthy F. D., Gore M. T., Marple D. N., Sartin J. L. Skeletal muscle protein synthesis and growth hormone secretion in young lambs treated with clenbuterol. J Anim Sci. 1989 Sep;67(9):2245–2254. doi: 10.2527/jas1989.6792245x. [DOI] [PubMed] [Google Scholar]
- Degroodt J. M., Wyhowski de Bukankski B., Beernaert H., Courtheyn D. Clenbuterol residue analysis by HPLC-HPTLC in urine and animal tissues. Z Lebensm Unters Forsch. 1989 Aug;189(2):128–131. doi: 10.1007/BF01332946. [DOI] [PubMed] [Google Scholar]
- Degroodt J. M., Wyhowski de Bukankski B., Beernaert H., Courtheyn D. Clenbuterol residue analysis by HPLC-HPTLC in urine and animal tissues. Z Lebensm Unters Forsch. 1989 Aug;189(2):128–131. doi: 10.1007/BF01332946. [DOI] [PubMed] [Google Scholar]
- Degroodt J. M., Wyhowski de Bukanski B., De Groof J., Beernaert H. Cimaterol and clenbuterol residue analysis by HPLC-HPTLC in liver. Z Lebensm Unters Forsch. 1991 May;192(5):430–432. doi: 10.1007/BF01193142. [DOI] [PubMed] [Google Scholar]
- Marsac J. H., Vlastos F. D., Lacronique J. G. Inhaled beta adrenergic agonists and inhaled steroids in the treatment of asthma. Ann Allergy. 1989 Sep;63(3):220–224. [PubMed] [Google Scholar]
- Martínez-Navarro J. F. Food poisoning related to consumption of illicit beta-agonist in liver. Lancet. 1990 Nov 24;336(8726):1311–1311. doi: 10.1016/0140-6736(90)92990-y. [DOI] [PubMed] [Google Scholar]
- Meyer H. H. The illegal practice and resulting risks versus the controlled use of licensed drugs: views on the present situation in Germany. Ann Rech Vet. 1991;22(3):299–304. [PubMed] [Google Scholar]
- Miller M. F., Cross H. R., Wilson J. J., Smith S. B. Acute and long-term lipogenic response to insulin and clenbuterol in bovine intramuscular and subcutaneous adipose tissues. J Anim Sci. 1989 Apr;67(4):928–933. doi: 10.2527/jas1989.674928x. [DOI] [PubMed] [Google Scholar]
- O'Connor B. J., Aikman S. L., Barnes P. J. Tolerance to the nonbronchodilator effects of inhaled beta 2-agonists in asthma. N Engl J Med. 1992 Oct 22;327(17):1204–1208. doi: 10.1056/NEJM199210223271704. [DOI] [PubMed] [Google Scholar]
- Pulce C., Lamaison D., Keck G., Bostvironnois C., Nicolas J., Descotes J. Collective human food poisonings by clenbuterol residues in veal liver. Vet Hum Toxicol. 1991 Oct;33(5):480–481. [PubMed] [Google Scholar]
- Weisbach W., Wagner F., Jäger K. H. Orale Langzeittokolyse der drohenden Frühgeburt mit Clenbuterol (Contraspasmin, Spiropent). Eine plazebokontrollierte Doppelblindstudie. Zentralbl Gynakol. 1986;108(7):419–423. [PubMed] [Google Scholar]
- Williams P. E., Pagliani L., Innes G. M., Pennie K., Harris C. I., Garthwaite P. Effects of a beta-agonist (clenbuterol) on growth, carcass composition, protein and energy metabolism of veal calves. Br J Nutr. 1987 May;57(3):417–428. doi: 10.1079/bjn19870049. [DOI] [PubMed] [Google Scholar]


