Abstract
We have used comprehensive synthetic lethal screens and biochemical assays to examine the biological role of the yeast amphiphysin homologues Rvs161p and Rvs167p, two proteins that play a role in regulation of the actin cytoskeleton, endocytosis, and sporulation. We found that unlike some forms of amphiphysin, Rvs161p-Rvs167p acts as an obligate heterodimer during vegetative growth and neither Rvs161p nor Rvs167p forms a homodimer in vivo. RVS161 and RVS167 have an identical set of 49 synthetic lethal interactions, revealing functions for the Rvs proteins in cell polarity, cell wall synthesis, and vesicle trafficking as well as a shared role in mating. Consistent with these roles, we show that the Rvs167p-Rvs161p heterodimer, like its amphiphysin homologues, can bind to phospholipid membranes in vitro, suggesting a role in vesicle formation and/or fusion. Our genetic screens also reveal that the interaction between Abp1p and the Rvs167p Src homology 3 (SH3) domain may be important under certain conditions, providing the first genetic evidence for a role for the SH3 domain of Rvs167p. Our studies implicate heterodimerization of amphiphysin family proteins in various functions related to cell polarity, cell integrity, and vesicle trafficking during vegetative growth and the mating response.
INTRODUCTION
RVS161 and RVS167, which encode closely related proteins in Saccharomyces cerevisiae, were first identified in a screen for mutants that exhibited reduced viability upon starvation (Bauer et al., 1993). Mutation of RVS161 or RVS167 causes a phenotype consistent with a role for the Rvs proteins in cortical actin cytoskeleton organization and endocytosis: loss of viability and unusual cell morphology in poor growth medium or salt-containing medium, delocalized actin distribution under suboptimal growth conditions, abnormal (random) budding in diploids, and defects in endocytosis and sporulation (Bauer et al., 1993). Ultrastructural studies have revealed that rvs mutants accumulate late secretory vesicles at sites of membrane and cell wall construction (Breton et al., 2001), suggesting an additional role for Rvs161p and Rvs167p in vesicle trafficking.
Rvs161p and Rvs167p are members of the BAR-domain family of proteins, which includes Bin1, Amphiphysin, and Rvs proteins (Sivadon et al., 1997). Amphiphysins are enriched in the mammalian brain and seem to function in synaptic vesicle endocytosis (for review, see Zhang and Zelhof, 2002). Bin1 is a splice isoform of amphiphysin 2 that has features of a tumor suppressor (Sakamuro et al., 1996). Proteins in this family are characterized by the presence of a conserved BAR domain, and it is through their BAR domains that Rvs161p interacts with Rvs167p (Navarro et al., 1997; Sivadon et al., 1997; Colwill et al., 1999). The crystal structure of the BAR domain of Drosophila amphiphysin has recently been solved (Peter et al., 2004). It is a crescent-shaped dimer, in which each monomer forms a coiled coil. The curved shape is only revealed upon dimerization of the two slightly kinked monomers, suggesting that the domain functions only as a dimer. The BAR domain binds preferentially to highly curved negatively charged membranes in vitro, and this property is thought to promote membrane deformation, leading to vesicle formation (Lee and Schekman, 2004; Peter et al., 2004). The central portion of Rvs167p consists of a region rich in glycine, proline, and alanine (the GPA region) and may play a role in Rvs regulation because it is phosphorylated in vivo (Friesen et al., 2003). At its carboxy terminus, Rvs167p, like the amphiphysins, has a Src homology 3 (SH3) domain (Figure 1A), a protein module well defined for binding proline-rich sequences (Pawson and Scott, 1997). Large-scale two-hybrid and phage-display screens have identified a number of proteins that bind to the SH3 domain of Rvs167p (Bon et al., 2000; Uetz et al., 2000; Drees et al., 2001; Ito et al., 2001; Tong et al., 2002); however, few of these interactions have been confirmed. Domain mapping of Rvs167p has revealed that the GPA region and the SH3 domain are largely dispensable for all Rvs167p functions tested (Navarro et al., 1997; Colwill et al., 1999). Thus, although the SH3 domain is conserved among amphiphysins, and several biologically important ligands have been seen to bind to the SH3 domain, its biological function remains unknown.
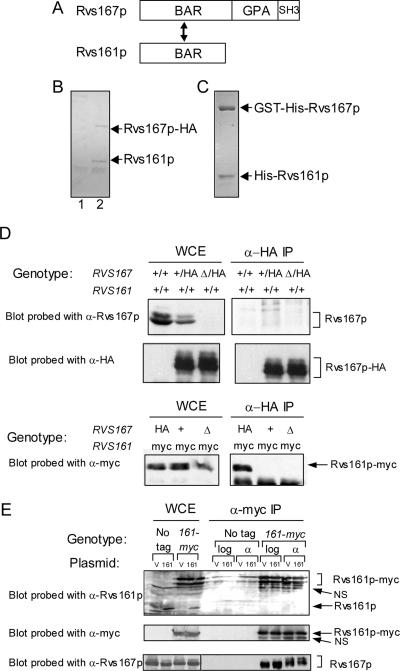
Figure 1.
Interaction of Rvs167p and Rvs161p. (A) Schematic illustration of the domain structures of Rvs167p and Rvs161p. The relative positions of the N-terminal BAR domain, the GPA region, and the C-terminal SH3 domain in Rvs167p and the BAR domain of Rvs161p are indicated. Rvs167p and Rvs161p interact with each other through their BAR domains. (B) Coimmunoprecipitation of Rvs167p-HA and Rvs161p from yeast extracts. Coomassie blue-stained gel showing α-HA immunoprecipitates from an untagged yeast strain, BY263 (lane 1), and from a yeast strain expressing RVS167-HA, BY1034 (lane 2). The lower molecular weight band was cut out of the gel, processed for mass spectrometry, and identified as Rvs161p (see Materials and Methods). The positions of migration of Rvs167p-HA and Rvs161p are indicated on the right. (C) Copurification of GST-His-Rvs167p and His-Rvs161p proteins coexpressed in insect cells. GST-His-Rvs167p and associated His-Rvs161p were purified using glutathione-Sepharose beads and analyzed by SDS-PAGE followed by staining with Coomassie blue. (D) Assay for homodimerization of Rvs167p. Lysates from diploid strains with either two untagged copies of RVS167, BY264 (lane 1), one untagged and one tagged copy of RVS167, BY1910 (lane 2), or one tagged copy of RVS167 and one deleted copy of RVS167, BY2591 (lane 3) were incubated with anti-HA antibodies. Immunoprecipitated proteins were analyzed by SDS-PAGE and immunoblotting with antibodies against Rvs167p (top) and anti-HA antibodies (middle). The anti-Rvs167p antibody does not detect HA-tagged Rvs167p. Lysates from haploid strains expressing RVS167-HA and RVS161-MYC, BY2592 (lane 4), RVS167-HA only, BY1034 (lane 5), or deletion of RVS167 and RVS161-MYC, BY2502 (lane 6) were incubated with antibodies against HA. The immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with anti-myc antibodies (bottom). (E) Assay for homodimerization of Rvs161p. Lysates from haploid yeast strains (either untagged, BY263, or with RVS161-myc, BY1182) bearing a plasmid encoding untagged Rvs161p were analyzed by coimmunoprecipitation and Western blot. Cells were grown to midlog phase and then treated or mock treated with 5 μM α factor for 1 h. The positions of migration of relevant proteins is marked on the right; NS, nonspecific band.
Several lines of evidence support a model in which Rvs161p and Rvs167p function together. First, Rvs161p and Rvs167p interact with each other through their respective BAR domains in two-hybrid assays, and Rvs161p can be immunoprecipitated from cells overproducing tagged Rvs167p (Navarro et al., 1997; Colwill et al., 1999). Second, each RVS gene is required for the stability of its Rvs protein partner during vegetative growth in vivo (Lombardi and Riezman, 2001). Third, the phenotype of an rvs161Δ mutant seems identical to that of an rvs167Δ mutant in vegetative cells (Crouzet et al., 1991; Bauer et al., 1993; Sivadon et al., 1995), and the extragenic suppressors sur1Δ, sur2Δ, and sur4Δ (Desfarges et al., 1993), fen1Δ, and ipt1Δ (Balguerie et al., 2002) and the multicopy suppressor SUR7 (Sivadon et al., 1997) are able to bypass the defects of either an rvs161Δ or rvs167Δ strain (Desfarges et al., 1993; Sivadon et al., 1997).
Nonetheless, evidence for distinct roles for the Rvs proteins has also been reported. The BAR domains of Rvs161p and Rvs167p cannot be functionally exchanged with each other, showing that, despite being structurally similar, this domain confers specificity to the activity of each Rvs protein (Sivadon et al., 1997). Rvs167p homodimerization has also been reported (Colwill et al., 1999; Bon et al., 2000; Lombardi and Riezman, 2001). Furthermore, the localization patterns of Rvs161p and Rvs167p do not completely overlap: Rvs167p is localized to cortical actin patches (Balguerie et al., 1999), whereas Rvs161p is reportedly mainly cytoplasmic with small dots distributed randomly within the cell cortex in unbudded cells and at the mother-bud neck during bud emergence and cytokinesis (Brizzio et al., 1998), although Rvs161p-green fluorescent protein has also been localized to small cortical patches during G1 (Balguerie et al., 2002). Finally, synthetic mating defects in combination with fus1Δ are seen with rvs161Δ but not rvs167Δ, revealing a specific role for Rvs161p in cell fusion during mating (Brizzio et al., 1998).
In this study, we have used genetic analyses of RVS161 and RVS167 to ask whether Rvs161p and Rvs167p have separate functions in vivo and to characterize the roles of the Rvs proteins. Comprehensive synthetic lethal screens and biochemical studies have identified shared roles for Rvs161p and Rvs167p in cell polarity, cell integrity, cell wall synthesis, and vesicle trafficking, all processes that require proper formation and fusion of vesicles. Consistent with these biological roles, we found that purified Rvs161p-Rvs167p is able to bind to phospholipid vesicles, as is seen with its Drosophila and mammalian homologues. We have also devised specific genetic screens that uncovered a previously unappreciated role for Rvs167p in mating and an essential role for the Rvs167p SH3 domain in some strain backgrounds. We conclude that Rvs161p and Rvs167p function as an obligate heterodimer in vegetative cells and have partially overlapping roles during mating.
MATERIALS AND METHODS
Strains and Media
S. cerevisiae strains used in this study are listed in Table 1. Strains were obtained from the yeast gene-deletion mutant collection constructed by the deletion consortium (Winzeler et al., 1999) or were constructed using standard yeast genetic techniques (Sherman, 1991). Deletions of open reading frames (ORFs) were constructed by integrative transformation (Longtine et al., 1998). Standard methods and media were used for yeast growth and transformation (Guthrie and Fink, 1991). Supplemented minimal medium containing 2% dextrose (SD) or 2% galactose (SG) with appropriate amino acid supplements was used to select for plasmids. Salt-containing medium was made by adding NaCl to synthetic dextrose medium or YPD to a final concentration (wt/vol) as indicated. Sorbitol was added to minimal synthetic medium containing glucose or galactose to a final concentration of 1 M to maintain rvs167Δ slt2Δ mutants. Where indicated, α-factor (obtained from the Louisiana State University Health Sciences Center Core Laboratory, New Orleans, LA) was added to log-phase cultures in YPD to a final concentration of 5 μM, and cells were incubated as indicated before harvesting. For spot assays, cultures grown to mid-log phase in SD were serially diluted 10-fold and spotted onto the appropriate plates.
Table 1.
Yeast strains used in this study
| Strain | Reference | |
|---|---|---|
| BY263a | MATatrp1Δ63 GAL2+ ura3-52 lys2-801am ade2-107o his3Δ200 leu2-Δ1 | Measday et al. (1994) |
| BY1034a | BY263 RVS167-HA::kan | This study |
| BY264a | MATa/α trp1Δ63/trp1Δ63 GAL2/gal2 ura3-52/ura3-52 lys2-801/lys2-801 ade2-107o/ade2-107o his3Δ200/his3Δ200 | Measday et al. (1994) |
| BY1910a | BY264 RVS167/RVS167-HA::kan | This study |
| BY2591a | BY264 rvs167Δ::TRP1/RVS167-HA::kan | This study |
| BY2592a | BY263 RVS167-HA::kan RVS161-myc::kan | This study |
| BY2502a | BY263 rvs167Δ::TRP1 RVS161-myc::kan | This study |
| BY1182a | BY263 RVS161-MYC::kan | This study |
| BY4741ab | MATahis3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 met15Δ0 | Brachmann et al. (1998) |
| BY1404b | MATα mfa1ΔMFA1pr-HIS3 can1Δ his3Δ1 leu2Δ0 lys2Δ0 rvs167Δ::Nat | Friesen et al. (2003) |
| BY1796b | MATα mfa1ΔMFA1pr-HIS3 can1Δ his3Δ1 leu2Δ0 lys2Δ0 rvs161Δ::Nat | Tong et al. (2004) |
| b | BY4741a rvs167Δ::kan | Deletion consortium |
| b | BY4741a rvs161Δ::kan | Deletion consortium |
| BY2504c | MATα mfa1ΔMFA1pr-HIS3 can1Δ his3Δ1 leu2Δ0 lys2Δ0 rvs167Δ::URA3 | This study |
| BY1690c | MATα mfa1ΔMFA1pr-HIS3 can1Δ his3Δ1 leu2Δ0 lys2Δ0 rvs161Δ::URA3 | This study |
| BY2001c | MATα mfa1ΔMFA1pr-HIS3 can1Δ his3Δ1 leu2Δ0 lys2Δ0 sla1Δ::Nat | This study |
| BY2102b | BY4741 MATα end3Δ::kan | This study |
| BY1891b | MATaslt2Δ::kan | Deletion consortium |
| BY2506b | MATarvs167Δ::Nat slt2Δ::kan | This study |
| BY2805b | MATarvs167Δ::Nat sla1Δ::kan | This study |
| BY2806b | MATaabp1Δ::Nat sla1Δ::kan | This study |
BY263-based; BY263 is an ssd1-d strain derived from S288C.
BY4741-based; BY4741 is an SSD1-V strain derived from S288C.
BY4741-based query strain for SGA.
Plasmids
Plasmids are described in Table 2. Details of construction of all plasmids are available upon request. The ABP1ΔPXXP (BA1732) plasmid was constructed with the use of PCR with Platinum Pfx (Invitrogen, Carlsbad, CA). Two PCR products were synthesized, using a plasmid containing ABP1 under the control of its own promoter as template. Each PCR product introduced an NheI site within the ABP1 coding sequence (the sequence of the primers is available upon request), one at each end of the proline-rich region. The 5′-PCR product was digested with NheI and BamHI and the 3′-PCR product was digested with NheI and ApaI. These were ligated into pRS315 (Sikorski and Hieter, 1989) that had been digested with BamHI and ApaI, giving pRS315-ABP1ΔPXXP, which contained an in-frame deletion of amino acids 448–525. The integrity of all PCR products was confirmed by sequencing.
Table 2.
Plasmids used in this study
| Plasmid | Description | Source | |
|---|---|---|---|
| BA1533 | pHis-RVS161 | RVS161 ORF with an N-terminal 6-His-tag subcloned in pAcHLT-C for baculoviral expression | This study |
| BA1534 | pRVS167-HIS-GST | RVS167 ORF subcloned in pAcGHLT-C for baculoviral expression | Friesen et al. (2003) |
| BA1766 | pRS316-RVS161 | Genomic fragment of RVS161 in low copy plasmid | This study |
| BA1387 | p416MET-RVS167 | RVS167 ORF under MET25 promoter in low copy plasmid | Friesen et al. (2003) |
| BA1840 | pRS426-RVS161 | Genomic fragment of RVS161 in high copy plasmid | This study |
| BA1658 | p416MET-RVS167-P473L | RVS167 ORF with P473L substitution in SH3 domain under MET25 promoter in low copy plasmid | This study |
| BA1437 | p416MET-RVS167-4A | RVS167 ORF with S299A, S321A, T323A, S379A substitutions under MET25 promoter in low copy plasmid | Friesen et al. (2003) |
| BA1019 | pSLT2-HA | SLT2-3HA fusion on high copy plasmid | Madden et al. (1997) |
| BA1422 | pGAL-GST-MNN9 | MNN9 ORF fused to GST-His at N-terminus under GAL1 promoter on high copy plasmid | Zhu et al. (2000) |
| BA1577 | pGAL-CCW12 | CCW12 ORF under GAL promoter on high copy plasmid | This study |
| BA1695 | pRS316-ABP1ΔSal | Genomic fragment of ABP1 on low copy plasmid | This study |
| BA1732 | pRS315-ABP1ΔPXXP | Genomic fragment of ABP1 encoding a protein with a deletion of the PXXP-containing region (codons 448-525) on low copy plasmid | This study |
| BA1697 | pRS315-ABP1 | Genomic fragment of ABP1 on low copy plasmid | This study |
Protein Immunoprecipitation and Western Blotting
Yeast strains were grown at 30°C to log phase, harvested, and lysed by vortexing with glass beads in lysis buffer as described previously (Tennyson et al., 1998). Where specified, α-factor was added to 5 μM and the cultures treated as described in figure legends. For large-scale immunoprecipitation of Rvs167p-hemagglutinin (HA), log-phase cells were harvested in immunoprecipitation-lysis buffer (50 mM Tris-Cl, pH 7.4, 250 mM NaCl, 50 mM NaF, 0.1% NP-40, 40 mM β-glycerophosphate, 5 mM EDTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 0.1 mg/ml insulin, and protease inhibitor tablet without EDTA; Boehringer Mannheim, Laval, Quebec, Canada). Cells were lysed by vortexing in the presence of glass beads. One hundred milligrams of lysate was precleared with 300 μl of protein A-Sepharose for 1 h and then incubated with 200 μl of α-HA antibodies coupled to protein A-Sepharose for 1 h. The beads were then washed in 3 × 5 ml lysis buffer and boiled in SDS sample buffer. For Western blotting of whole cell extract, the protein concentration of the lysates was measured using the Bradford assay (Bio-Rad, Mississauga, Ontario, Canada). Equivalent amounts of each sample were analyzed by SDS-PAGE and transferred to nitrocellulose using a dry transfer apparatus. Coimmunoprecipitations were done as described previously (Friesen et al., 2005). Immunoblotting was done with antibodies against Rvs161p (gift from H. Riezman, University of Geneva, Geneva, Switzerland), Rvs167p (Lee et al., 1998) and HA (12CA5). As a control, antibodies against Crn1p were used (Humphries et al., 2002). The protein signal was revealed using enhanced chemiluminescence (Amersham Biosciences, Little Chalfont, Buckinghamshire, United Kingdom).
Slt2p-HA was immunoprecipitated with monoclonal antibody 12CA5 (Wilson et al., 1984) from 100-ml cultures either grown at 24°C or grown at 24°C and transferred to 39°C for 2 h. Cells were collected by centrifugation, and lysates were prepared as described previously (Tennyson et al., 1998). Five milligrams of protein from the lysates were incubated with 1 μl of 12CA5 ascitic fluid for 1 h on ice and then rocked in the presence of protein A-Sepharose for 1 h at 4°C. Beads were collected by centrifugation and washed in lysis buffer four times.
Expression and Purification of Rvs167p and Rvs161p from Insect Cells
A BamHI fragment containing full-length RVS161 was subcloned into pAC-HLT (BD Biosciences PharMingen, San Diego, CA) that had been digested with BglII to generate a His-tagged Rvs161p fusion product. A BamHI fragment containing full-length RVS167 was subcloned into pAC-GHLT (BD Biosciences PharMingen) that had been digested with BglII to generate a glutathione S-transferase (GST)-His-tagged Rvs167p fusion product as described previously (Friesen et al., 2003). Recombinant baculoviruses containing RVS161 and RVS167 were isolated from Sf9 insect cells and coinfected into Hi5 cells as recommended by the manufacturer (Invitrogen). Approximately 48 h after infection, cells were harvested, washed, and lysed in insect cell lysis buffer (50 mM Tris-HCl, 20% glycerol, 50 mM NaCl, 0.5 mM EDTA, and 10 mM β-mercaptoethanol). Cell extracts were incubated with glutathione-Sepharose beads (Amersham Biosciences) for 1 h at 4°C. Beads were washed 4 times in 10 volumes of lysis buffer, and GST-His-Rvs167p and associated His-Rvs161p were eluted with lysis buffer containing 100 mM glutathione.
Synthetic Lethality Screens and Complementation Assays
We used the synthetic genetic array (SGA) method to systematically identify mutant backgrounds in which RVS167 or RVS161 is required for viability or wild-type growth at 30°C (Tong et al., 2001, 2004). All interactions were confirmed by tetrad dissection. Nonoverlapping interactions between the RVS161 and RVS167 screens were tested against both rvs deletions. Because the frequency of false negatives in SGA analysis can be as high as 40% (Tong et al., 2004), we also tested candidate genes encoding members of a protein complex for interactions with our query genes if genes encoding two or more members of the protein complex were identified to have synthetic interactions (e.g., members of the prefoldin complex). The data for the RVS161 and RVS167 screens have been published previously (Tong et al., 2004).
Complementation of the sla1Δ abp1Δ strain was assayed using the plasmid shuffle method. The sla1Δ abp1Δ strain containing the URA3-based plasmid pRS316-ABP1 was transformed with the LEU2-based plasmid pRS315-ABP1 or pRS315-ABP1-ΔPXXP. Double transformants were pregrown on SD-Leu, and then cells that had lost the URA3 plasmid were selected on 5-fluoroorotic acid (5-FOA) and assayed by spot dilution on YPD at 37°C and YPD + 3 mM caffeine at 35°C.
Synthetic Mating Defects Screen and Mating Assays
SGA screens were done to construct MATa double mutants that had rvs161Δ marked with URA3 (rvs161Δ::URA3 xxxΔ::kan) (see above; Tong et al., 2001). To identify genes that were important for mating in the absence of RVS161, the ∼5000 viable MATa rvs161Δ::URA3 xxxΔ::kan mutants were crossed to a MATα strain in which the RVS161 ORF had been replaced with the gene encoding nourseothricin resistance (rvs161Δ::Nat) by replica pinning onto YPD plates. The cells were allowed to mate for 6 h at 30°C and then replica pinned onto YPD plates containing nourseothricin (Nat) and G418 to select for diploid cells. Strains that had apparent synthetic mating defects in combination with rvs161Δ were retested in limited plate matings with rvs161Δ essentially as described previously (Brizzio et al., 1998). In brief, cells of each mating type were patched on top of each other on YPD plates. The plates were incubated at 30°C and replica plated after 2, 3, and 4 h to YPD + G418 + Nat to select for diploids. Likewise, limited mating assays of other strains were done by crossing a MATα rvs167Δ::Nat strain with MATa rvs167Δ::URA3 fus1Δ::kan, rvs167Δ::URA3 fus2Δ::kan, rvs167Δ::URA3 prm9Δ::kan, and rvs167Δ::URA3 fig2Δ::kan strains; a MATα sla1Δ::Nat strain with MATa sla1Δ::URA3 fus1Δ::kan, sla1Δ::URA3 fus2Δ::kan, sla1Δ::URA3 prm9Δ::kan, and sla1Δ::URA3 fig2Δ::kan strains; and a MATα end3Δ::Nat strain with MATa end3Δ::URA3 fus1Δ::kan, end3Δ::URA3 fus2Δ::kan, end3Δ::URA3 prm9Δ::kan, and end3Δ::URA3 fig2Δ::kan strains.
Fluorescence Microscopy
Ten milliliters of cells were grown to mid-log phase in YPD, fixed in 4% formaldehyde for 1.5 h with shaking, and then washed two times with phosphate-buffered saline (PBS), resuspended in 100 μl of PBS, and stored at 4°C. Thirty-six microliters of fixed cells were incubated with 4 μl of rhodamine-phalloidin (Invitrogen), 0.5 μl of 10% Triton X-100, and 4 μl of 1 mg/ml Fluorescent Brightener (also known as Calcofluor White; Sigma-Aldrich, St. Louis, MO) for 1.5 h in the dark with occasional gentle vortexing. Cells were washed three times in PBS, visualized using VectaShield mounting medium containing 4,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA), and viewed through fluorescein isothiocyanate and rhodamine filters. Photographs were taken with a Micromax 1300y high-speed digital camera (Princeton Instruments, Trenton, NJ) mounted on a Leica DM-LB microscope. Images from the camera were analyzed with MetaVue software (Universal Imaging, Media, PA).
Overexpression of MNN9 and CCW12 in an rvs167Δ slt2Δ Strain
Strains BY1891 (slt2Δ) and BY2506 (rvs167Δ slt2Δ) were transformed with pGAL-ORFs or empty vector, and BY2506 was kept alive on selective synthetic medium plus glucose and 1 M sorbitol at 30°C. Cells were then replica plated onto synthetic selective media plus 2% galactose and 1 M sorbitol, to induce expression of the pGAL-ORF. Ten-fold serial dilutions were made onto SD and SG containing 1 M sorbitol, and plates were incubated at 30°C to assess the growth of the cells.
Actin Binding Assay
Actin filament binding assays was performed as described previously (Goode et al., 1999). Purified Rvs167-GPA-SH3p-His (2.5 μM; Friesen et al., 2003) or Crn1p (2.5 μM; Humphries et al., 2002) was added to preassembled yeast actin filaments (5 μM) and incubated for 15 min at 25°C. The reactions were centrifuged at 90,000 rpm for 15 min in a TLA100.3 rotor, and the supernatants and pellets were analyzed by SDS-PAGE and Coomassie blue staining.
Liposome Sedimentation Assays
Purified GST-Rvs167p-Rvs161p was subjected to centrifugation at 140,000 × g for 30 min to remove insoluble material. Soluble Rvs167p-Rvs161p heterodimer (7 nM) was incubated with 0.8 mg/ml liposomes from total bovine brain lipids (Avanti Polar Lipids, Alabaster, AL; prepared as described by Peter et al., 2004) or buffer for 30 min at 25°C and then centrifuged at 140,000 × g for 20 min as described previously (Peter et al., 2004). After centrifugation, supernatants were removed immediately and pellets were resuspended in an equal volume of buffer. Samples of pellet and supernatant were analyzed by PAGE and Western blot with α-Rvs167p antibodies.
RESULTS
Rvs167p and Rvs161p Interact as a Heterodimer In Vivo and In Vitro
The structure of the BAR domain of the Drosophila amphiphysin protein suggests that BAR domain proteins are likely active only as dimers (Peter et al., 2004). Drosophila amphiphysin and other mammalian amphiphysins form homodimers; however, heterodimers have been observed for some members of the BAR domain family, for example, rat amphiphysin 1 and 2 (Wigge et al., 1997). A search of the SMART database (Letunic et al., 2004) reveals that Rvs161p and Rvs167p are the only BAR domain-containing proteins in S. cerevisiae. Thus, if dimerization is required for BAR-domain activity, the Rvs proteins must act as either an obligate heterodimer or they must homodimerize. Several laboratories have detected a two-hybrid interaction between the BAR domains of Rvs161p and Rvs167p (Navarro et al., 1997; Colwill et al., 1999; Lombardi and Riezman, 2001; Figure 1A), and Rvs161p has been found to coimmunoprecipitate with overproduced Rvs167p (Navarro et al., 1997). To test whether Rvs proteins expressed from their endogenous loci interact and to assay for other proteins that interact with Rvs167p at a stoichiometric level, we used an immunoaffinity purification procedure to isolate Rvs167p–HA complexes from yeast extracts (Figure 1B). By Coomassie blue staining, the only protein that immunoprecipitated with Rvs167p-HA was a protein of ∼30 kDa. Mass spectrometric analysis of peptides derived from the 30-kDa band indicated that the protein was Rvs161p (our unpublished data). The similar recovery of Rvs161p and Rvs167p in the Rvs167p-HA immune complexes suggests that Rvs161p-167p may form a stoichiometric complex in vivo.
In both rvs161Δ and rvs167Δ mutants, the Rvs protein levels are reduced because of reduced stability of one Rvs protein in the absence of the other (Lombardi and Riezman, 2001). This suggests that the interaction between Rvs161p and Rvs167p is required for their stability. Based on our finding that Rvs161p and Rvs167p interact in vivo, we thought that their interaction might function to stabilize each component of the complex in a heterologous system. Consistent with the requirement for an Rvs161p–167p interaction, we found that proteins encoded by either His-RVS161 or GST-His-RVS167 was largely insoluble when expressed (separately) in insect cells (Friesen et al., 2003; our unpublished data), and His-Rvs161p and GST-Rvs167p were insoluble when produced (separately) in Escherichia coli (our unpublished data), suggesting that the protein products may not fold properly in the absence of their partner (Colwill et al., 1999; Maxwell et al., 1999). To test whether the interaction between the Rvs proteins might allow production of a stable protein complex, we coinfected insect cells with baculoviral vectors that expressed both GST-His-RVS167 and His-RVS161. Cell lysates from coinfected cells were bound to glutathione-Sepharose columns, and both Rvs167p and Rvs161p were detected in the column eluates (Figure 1C). Thus, when coexpressed, Rvs167p and Rvs161p were able to form a soluble protein complex. These biochemical data are consistent with the hypothesis that Rvs167p and Rvs161p must function together in vivo.
Several laboratories have reported a two-hybrid interaction between the BAR domain of Rvs167p and full-length Rvs167p (Colwill et al., 1999; Bon et al., 2000; Lombardi and Riezman, 2001). However, a two-hybrid assay does not address whether homodimerization of Rvs167p occurs in vivo. We tested for Rvs167p homodimerization in a coimmunoprecipitation assay using a diploid strain expressing two alleles of RVS167, one encoding an HA-tagged version of Rvs167p and the other encoding an untagged Rvs167p protein. We found that Rvs167p did not coimmunoprecipitate with Rvs167p-HA, showing that homodimerization of Rvs167p does not occur in log-phase cells (Figure 1D, top). To confirm that the epitope-tagged Rvs167p was functional (i.e., able to bind Rvs161p), we showed that Rvs167p could immunoprecipitate an Rvs161p-myc fusion protein (Figure 1D, bottom). These data suggest that previous two-hybrid interactions between the BAR domain of Rvs167p and full-length Rvs167p may have been indirect or an artifact of BAR domain overproduction. In support of this, Bon et al. (2000) saw a two-hybrid interaction between Rvs167p and Rvs167p when both genes were on high-copy plasmids but not when they were on low-copy plasmids.
We also tested whether Rvs161p could homodimerize. Because Rvs161p and Rvs167p are the only BAR-domain-containing proteins in yeast and because Rvs161p is required for fusion during mating and Rvs167p is not (Brizzio et al., 1998), we were especially interested in whether Rvs161p would form homodimers in cells during the mating response. To test for Rvs161p homodimerization, we constructed a strain expressing RVS161-myc from its endogenous locus and transformed it with a plasmid containing an untagged version of RVS161. As a negative control, we used an untagged strain containing the RVS161 plasmid. We immunoprecipitated Rvs161p-myc with anti-myc antibodies and assayed for the presence of Rvs161p. As a positive control, we assayed for coimmunoprecipitation of Rvs167p. Although Rvs167p efficiently coimmunoprecipitated with Rvs161p-myc, Rvs161p did not immunoprecipitate with Rvs161p-myc, either in log-phase cells or in cells treated with α factor (Figure 1E). Together, these data show that Rvs161p is the predominant protein partner for Rvs167p in vivo and that these proteins do not form homodimers. These data strongly suggest that the Rvs161p-Rvs167p heterodimer is the functional unit of the Rvs proteins in vivo.
RVS161 and RVS167 Are Genetically Identical as Revealed by Analysis of Synthetic Lethality
To test whether the heterodimer of Rvs161p and Rvs167p accounts for all the cellular functions of the Rvs proteins, we looked at whether one Rvs protein had a function in the absence of its Rvs partner. We predicted that if Rvs161p and Rvs167p function only as a heterodimer, deletion of one RVS gene would be genetically identical to deletion of the other. To test this, and to explore the biological roles of Rvs161p and Rvs167p, we screened for genes that had synthetic genetic interactions with RVS161 and RVS167 by using SGA analysis. Double mutant strains that failed to grow, or that grew more slowly than wild type, identified candidate genetic backgrounds in which RVS was required. All the interactions with either RVS167 or RVS161 were confirmed by tetrad analysis in crosses with both rvs161Δ and with rvs167Δ mutants. The complete data set from these screens has been published previously (Tong et al., 2004).
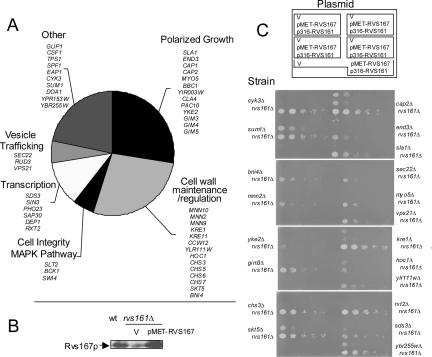
We found 49 strains whose deletion caused death or synthetic growth defects in combination with deletion of RVS167 (Figure 2A). All of these mutant strains (and no others) also showed synthetic lethal or synthetic growth defects with a deletion of RVS161. Genes that are required in the absence of RVS161 and RVS167 were organized into categories according to their roles as defined by the Yeast Proteome Database (https://proteome.incyte.com/control/tools/proteome), the Saccharomyces Genome Database (http://www.yeastgenome.org), and published reports (Figure 2A). As expected from the phenotypes of rvs deletion mutants (Crouzet et al., 1991; Sivadon et al., 1995, 1997; Lee et al., 1998; Breton et al., 2001; Lombardi et al., 2001; Balguerie et al., 2002), the RVS interactions were enriched for genes with roles in cell polarity, cell wall synthesis, cell wall integrity, and vesicle trafficking.
Figure 2.
RVS161 and RVS167 have identical synthetic genetic interactions. (A) Pie chart summarizing the results of the rvs167Δ and rvs161Δ synthetic lethality screens with query strains BY1404 and BY1796. Genes were categorized according to their annotations in the Saccharomyces Genome Database (SGD), the Yeast Proteome Database (YPD), and/or on the basis of review of published studies concerning the genes. (B) Western blot showing levels of Rvs167 protein in wild-type and rvs161Δ cells containing either vector or p416-MET-RVS167. (C) Assay for suppression of the synthetic growth defect of slow-growing rvs161Δ xxxΔ mutant strains by overproduction of Rvs167p. Twenty slow-growing viable rvs161Δ xxxΔ mutant strains were transformed with vector (p416-MET; V), p416-MET-RVS167, and pRS316-RVS161 and assayed by serial dilution onto SD-Ura and SD-Ura + 3% NaCl. Only the SD-Ura + 3% NaCl plates are shown. Strains were arrayed as shown in the diagram at the top with five yeast strains transformed with three plasmids on each plate. For many of the double mutant strains, cells containing vector or p416-RVS167 were unable to grow on 3% NaCl so that only growth of cells containing p316-RVS161 is apparent.
The Pattern of Identical Synthetic Genetic Interactions with RVS161 and RVS167 Is Not Because of Destabilization of the Rvs Partner
Because each Rvs protein is required for the solubility and stability of its Rvs partner in vivo (Lombardi and Riezman, 2001; Figure 1C), it was possible that the identical SGA profiles for RVS161 and RVS167 reflected the decreased protein levels of the remaining Rvs protein. We next asked whether increasing the cellular concentration of Rvs167p to wild-type levels in the rvs161Δ double mutants (which we refer to as rvs161Δ xxxΔ strains) could suppress their growth defect. Expression of RVS167 from the MET25 promoter restores Rvs167p protein to wild-type levels in the rvs161Δ RVS167+ strain (Figure 2B). We predicted that if the rvs161Δ xxxΔ mutant strains were slow growing because Rvs167p was destabilized, heterologous expression of RVS167 should rescue the growth defects of the double mutants. We compared the ability of empty vector, p416-MET-RVS167 and p316-RVS161 (RVS161 under the control of its own promoter) to complement the growth defects of 20 slow-growing rvs161Δ xxxΔ mutants grown on medium containing 3% salt, which provides more stringent conditions to detect the subtle growth defects of some of the double mutants. We found that restoring levels of Rvs167p by expressing RVS167 failed to rescue the growth defects of these strains (Figure 2C). Weak suppression of the rvs161Δ xxxΔ slow growth phenotype by RVS167 was detected in the sum1Δ, skt5Δ, and ybr255wΔ double mutants, but in all cases the RVS161 plasmid complemented the growth defect of the rvs161Δ xxxΔ strains much more efficiently than the RVS167 plasmid (Figure 2C; our unpublished data). These data show that restoring Rvs167p protein to wild-type levels is not sufficient to restore wild-type growth in strains in which RVS161 is needed. Thus, the decreased protein levels of the Rvs partner are not sufficient to explain the overlap of the genetic profiles of strains deleted for RVS167 or RVS161.
Reduced Stability of Each Rvs Protein in the Absence of its Partner in Pheromone-treated Cells
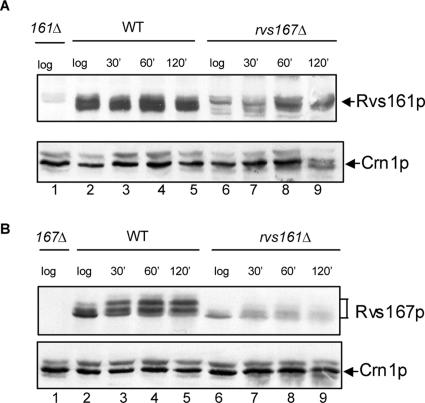
As noted above, previous studies showed that the levels of Rvs167p protein are reduced in an rvs161Δ mutant during log phase, and, similarly, the levels of Rvs161p are strongly reduced in an rvs167Δ mutant (Lombardi et al., 2001). The instability of the Rvs proteins in the absence of their partner is consistent with the model that they function together during vegetative growth. However, distinct phenotypes for rvs mutants have been reported during the mating response, and it is unclear whether the Rvs proteins function as a heterodimer during mating (Brizzio et al., 1998). We hypothesized that if Rvs167p has a role in mating, then Rvs161p protein levels might remain low in an rvs167Δ strain exposed to mating pheromone, despite the rapid induction of RVS161 expression in response to α factor (Brizzio et al., 1998). To test this, we used Western blots to look at the protein levels of Rvs161p and Rvs167p from extracts of cells treated with pheromone. Rvs161p protein levels were greatly decreased in extracts from rvs167Δ mutants in log phase compared with levels in wild-type cells (Figure 3A, compare lanes 2 and 6). On treatment with α factor, Rvs161p was detected at much lower levels in the rvs167Δ strain than in wild-type cells, even after incubation for 2 h in pheromone-containing medium (Figure 3A, lanes 7–9). In an analogous assay, we examined the protein levels of Rvs167p in rvs161Δ extracts after exposure to pheromone. Rvs167p protein levels were strongly reduced in log-phase rvs161Δ cells compared with wild-type cells and remained reduced during treatment with α factor (Figure 3B). These data reveal a requirement for the Rvs partner for the stability of the Rvs proteins during pheromone exposure, suggesting that the Rvs161p-Rvs167p heterodimer may function during mating.
Figure 3.
Reduced stability of Rvs161p and Rvs167p protein in the absence of its partner in log phase and during treatment with mating pheromone. (A) Western blot analysis of protein extracts from wild-type (BY4741a) and rvs167Δ cells in log phase and after 30-, 60-, and 120-min exposure to 5 μM α factor as indicated. Extracts were analyzed by SDS-PAGE and immunoblotted with anti-Rvs161p antibody (gift from H. Riezman). (B) Western blot analysis of protein extracts from wild-type and rvs161Δ cells in log phase and after 30-, 60-, and 120-min exposure to mating pheromone as indicated. Extracts were analyzed by SDS-PAGE and immunoblotted with anti-Rvs167p antibody. The same extracts were blotted with antibodies against Crn1p as a loading control (bottom). The position of migration of Rvs167p and Rvs167p phosphoforms, Rvs161p and Crn1p are indicated.
Identification of Mutations That Lead to Synthetic Mating Defects in Combination with Deletion of RVS161 and RVS167
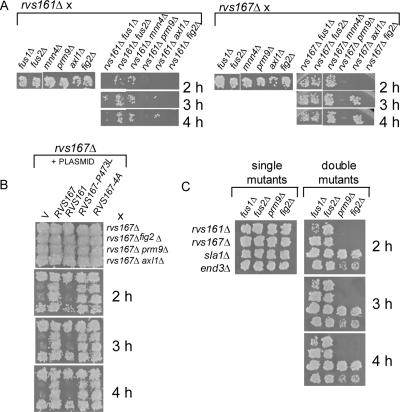
Our finding that stability of Rvs161p depends on Rvs167p during pheromone response suggests a function for both Rvs161p and Rvs167p during mating. However, only RVS161, but not RVS167, has been shown to be important for mating (Brizzio et al., 1998). The mating defects of a strain deleted for RVS161 alone are subtle but are exacerbated in combination with a deletion of FUS1 (Brizzio et al., 1998). We were therefore interested in identifying additional genetic backgrounds in which the mating defect of the rvs161Δ strain was exacerbated, to test whether a role for RVS167 in mating could be uncovered in these backgrounds. We used SGA to create double mutants of rvs161Δ in combination with the ∼5000 yeast gene deletions. These rvs161Δ xxxΔ mutants were then screened in limited mating assays for synthetic mating defects in crosses with an rvs161Δ mating partner using SGA (see Materials and Methods). We identified a number of genes that seemed to be required for mating in the absence of RVS161 (our unpublished data). We arbitrarily chose three genes, each of which has a role in cell fusion during mating: PRM9, AXL1, and FIG2. PRM9 encodes a pheromone-regulated membrane protein (Heiman and Walter, 2000), and its mutant phenotype has not yet been characterized. AXL1 encodes a protease required for cell fusion during mating (Elia and Marsh, 1998) and is required for axial budding (Fujita et al., 1994). FIG2 is involved in agglutination during mating (Zhang et al., 2002). We next tested whether some of these genes were required for mating in the absence of RVS167 using limited mating assays. We also included crosses with fus1Δ and fus2Δ mutants as positive and negative controls, because a strain with a deletion of RVS161 has mating defects in combination with fus1Δ but not fus2Δ (Brizzio et al., 1998), and mnn4Δ, another positive control, which had no defect in combination with rvs161Δ or rvs167Δ. All of these mutants showed no mating defect in an RVS+ background when crossed to rvs161Δ or rvs167Δ strains (Figure 4A, left). In an rvs161Δ background, a synthetic mating defect was seen in fus1Δ, prm9Δ, axl1Δ, and fig2Δ, but not in fus2Δ or in mnn4Δ (Figure 4A, right). In the rvs167Δ background, no mating defect was seen in fus1Δ, fus2Δ, axl1Δ, or in mnn4Δ strains, although the axl1Δ strain seemed to be delayed in mating (Figure 4A, right). But rvs167Δ prm9Δ and rvs167Δ fig2Δ strains had a mating defect similar to that seen in an rvs161Δ background. All these results were confirmed in quantitative mating assays (our unpublished data). We also found that the rvs161Δ and rvs167Δ double mutants (with fus1Δ, fus2Δ, prm9Δ, and fig2Δ) were able to form shmoos with >50% wild-type efficiency, indicating that their pheromone signaling pathway was functional, consistent with the defect in cell fusion previously reported in rvs161Δ strains. In summary, we found that rvs161Δ has synthetic mating defects with fus1Δ and axl1Δ that are not seen with rvs167Δ. Both rvs161Δ and rvs167Δ have defects in combination with deletions in prm9Δ and fig2Δ.
Figure 4.
Diploid selection plates from limited mating assays for rvs161Δ and rvs167Δ double mutants. (A) MATa rvs161Δ::URA3 xxxΔ::kan strains were mixed with a toothpick on a YPD plate with a MATα rvs161Δ::Nat strain, incubated at 30°C for 2, 3, and 4 h and replica plated to YPD + G418 + Nat to select for diploids. Likewise, MATa rvs167Δ::URA3 xxxΔ::kan strains were mated with a MATα rvs167Δ::Nat strain. On the left, for each strain, is shown a control plate of single mutant strains (e.g., fus1Δ::kan) crossed to rvs161Δ::Nat or rvs167Δ::Nat in which diploids were selected 2 h after mating. (B) MATa rvs167Δ::URA3 xxxΔ::kan strains transformed with p416-MET, p416-MET-RVS167, p426-RVS161, p416-MET-RVS167-P473L, or p416-MET-RVS167–4A were crossed with a MATα rvs167Δ::Nat strain as described above. (C) Limited-mating assay for mutants defective in endocytosis. MATa double mutants of rvs161Δ::URA3, rvs167Δ::URA3, sla1Δ::URA3, and end3Δ::URA3 in combination with fus1Δ::kan, fus2Δ::kan, prm9Δ::kan, and fig2Δ::kan were crossed to MATα rvs161Δ::Nat, rvs167Δ::Nat, sla1Δ::Nat, or end3Δ::Nat, incubated at 30°C for 2, 3, and 4 h and replica plated to YPD + G418 + Nat.
Because we had found that Rvs161p levels were significantly decreased during exposure to mating pheromone in an rvs167Δ strain (Figure 3A), one possibility was that the mating defect was because of destabilized Rvs161p and not absence of Rvs167p. To test this possibility, we assayed rvs167Δ cells transformed with a high copy plasmid containing RVS161 under its own promoter in a limited mating assay. As a positive control, we also tested rvs167Δ cells with a plasmid containing RVS167. In this experiment, RVS167 but not high copy RVS161 was able to complement the mating defect of the rvs167Δ xxxΔ mutants (Figure 4B). This suggests that the mating defect in rvs167Δ cells is not because of reduced stability of Rvs161p.
The BAR domain of Rvs167p is largely able to complement all the defects of an rvs167Δ strain during log-phase growth, but we were interested in whether the SH3 domain or the phosphorylation sites within the GPA region of Rvs167p were needed for mating. Rvs167p is hyperphosphorylated during the mating response (Lee et al., 1998; Friesen et al., 2003). We tested the rvs167Δ xxxΔ mutants for complementation using a plasmid that encodes Rvs167p with a mutant SH3 domain (Rvs167p-P473L). This Rvs167p SH3 mutant protein does not interact with the proline-rich region of Abp1 in a two-hybrid assay, but it does bind other proteins that interact with Rvs167p outside the SH3 domain, indicating that this mutant specifically disrupts the function of the SH3 domain (Colwill et al., 1999). We also tested for complementation using a plasmid with RVS167 encoding a protein with alanine substitutions at all four of the residues that are phosphorylated in vivo (Rvs167p-4A; Friesen et al., 2003). The rvs167Δ xxxΔ mutants were complemented equally efficiently by wild-type RVS167 and by RVS167-P473L and by RVS167-4A (Figure 4B). This indicates that neither an intact SH3 domain nor phosphorylation of Rvs167p is required for Rvs167p function during mating.
We were interested in whether the mating defects in rvs167Δ cells were characteristic of all endocytosis mutants. Brizzio et al. (1998) assayed several mutants defective in endocytosis for defects in mating and found that end3-1, end4-1, end6-1, and end7-1 behaved like wild type, suggesting that endocytosis did not play a role in cell fusion. However, it was possible that these defects could only be uncovered in certain specific genetic backgrounds, as we had seen for rvs167Δ. We tested two well-characterized endocytosis mutants, end3Δ (Raths et al., 1993) and sla1Δ (Warren et al., 2002), along with rvs161Δ and rvs167Δ, for mating in the absence of FUS1, FUS2, PRM9, and FIG2 (Figure 4C). As described above (Figure 4, A and B), rvs161Δ had a mating defect in the absence of FUS1, and rvs161Δ and rvs167Δ had mating defects in the absence of PRM9 and FIG2 (Figure 4C). In contrast, the sla1Δ strain and the end3Δ strain were able to mate in the absence of PRM9 and FIG2 (Figure 4C). Mating of the end3Δ strain was not as efficient as mating in the other strains, possibly because the end3Δ strain is slow growing by itself. Thus, in these assays, RVS167 has a role in mating that is partially distinct from that of RVS161 but is not found in other endocytosis mutants.
Synthetic Defects in Actin Organization and Chitin Distribution in Double Mutants
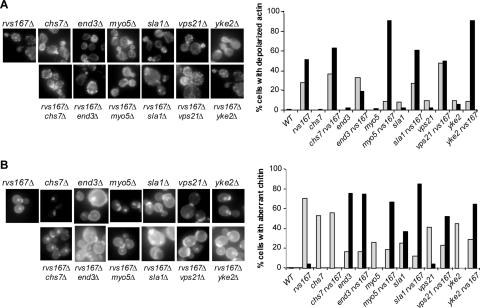
Identification of synthetic lethal interactions or synergistic growth defects provides support for a functional interaction between gene products (Bender and Pringle, 1991, Tong et al., 2004). Furthermore, the subtle defects in the organization and polarity of cortical actin structures of null mutations in cytoskeleton protein genes, and the large number of synthetic lethal interactions among these genes reveal their multiple overlapping functions (Ayscough and Drubin, 1996; Goode and Rodal, 2001). However, the cellular basis for the synthetic lethality is not always clear, and we decided to characterize the phenotypes of a subset of the mutants identified in our SGA analysis to determine whether polarity was defective in the double mutant strains. We first decided to examine the actin cytoskeleton. The rvs161Δ and rvs167Δ strains have partial defects in the polarization of their cortical actin cytoskeleton, suggesting their function in this process must be partially redundant with other cytoskeleton proteins (Sivadon et al., 1995; Lee et al., 1998; Balguerie et al., 1999). In a subset of the viable rvs167Δ xxxΔ mutants with synthetic growth defects and in the corresponding single mutants, we visualized filamentous actin using rhodamine-phalloidin staining (Figure 5A). Quantification of cells showing a defect in actin polarization revealed that rvs167Δ cells had a partially depolarized actin cytoskeleton (Figure 5A, left), as reported previously. In some of the rvs167Δ xxxΔ mutants, the defects in actin organization were no worse than in the single mutants. However, in the myo5Δ rvs167Δ and yke2Δ rvs167Δ double mutants cortical actin patches were seen randomly distributed over the periphery of both mother and daughter cell at all stages of the cell cycle (Figure 5A). This suggests that in these mutants the synergistic growth defect may be because of a depolarized actin cytoskeleton.
Figure 5.
Actin and chitin staining of single and double mutant cells of chs7Δ, end3Δ, myo5Δ, sla1Δ, vps21Δ, and yke2Δ in combination with rvs167Δ, as indicated above and below each panel. (A) Cells were grown in YPD to log phase, fixed and their actin stained using rhodamine-conjugated phalloidin as described in Materials and Methods. On the left are shown representative cells from each strain. On the right is shown a graph depicting percentage of cells with depolarized actin. Approximately 100 small- and medium-budded cells were scored as having: >90% of their actin in the bud (not graphed); 50–90% of their actin in the bud (gray); or <50% of their actin in the bud (black). (B) Cells were stained with Calcofluor White and DAPI, washed, and observed for chitin distribution. On the left are shown representative cells from each strain. On the right is shown a graph depicting percentage of cells with aberrant chitin distribution. Approximately 100 cells were scored for each strain as having: staining localized almost completely to the bud scars (not graphed), some diffuse chitin with easily discernible bud scars (gray), or completely diffuse chitin (black).
The rvs167Δ xxxΔ mutants were also examined for another phenotype that is often associated with perturbation of the actin cytoskeleton: cell wall defects. Mutations affecting actin can cause cell wall defects as indicated by depolarized chitin deposition (Novick and Botstein, 1985; Yang et al., 1997). We looked at cells stained with Calcofluor White to visualize the chitin deposited in the cell wall in bud scars and with DAPI to monitor position in the cell cycle. As reported previously (Bauer et al., 1993), the rvs167Δ mutant contained slightly diffuse chitin distributed over the entire mother cell surface (Figure 5B). Of the six rvs167Δ xxxΔ strains tested, rvs167Δ myo5Δ, rvs167Δ sla1Δ, rvs167Δ vps21Δ, and rvs167Δ yke2Δ showed a more diffuse chitin localization and weaker staining of bud scars than either of the single mutants (Figure 5B, quantification shown on the left). Interestingly, deletion of the genes with roles in endocytosis, MYO5 (Geli and Riezman, 1996), SLA1 (Warren et al., 2002), and VPS21 (Singer-Krüger et al., 1994), in combination with deletion of RVS167, resulted in cell wall defects. Thus, although rvs167Δ mutants have only partial defects in their actin cytoskeleton and cell wall, one or both of these defects were made more severe in several of the double mutants tested. Together, these data show that the growth defects of rvs167Δ double mutants may be caused by synergistic defects in cell wall structure and in the organization of the actin cytoskeleton.
The Cell Integrity Pathway Is Activated in rvs Mutants
The Slt2p kinase becomes phosphorylated on T190 and Y192 upon activation of the Slt2p MAP kinase pathway during periods in which yeast cells are undergoing polarized cell growth, namely, during bud formation and in response to mating pheromone and in response to environmental conditions such as cell wall damage and heat stress (Zarzov et al., 1996; de Nobel et al., 2000). Based on the observations that rvs161Δ and rvs167Δ mutants have defects in their cell wall (Figure 5B) and synthetic lethal interactions with genes that encode components of the Slt2p pathway (Figure 2A; Breton et al., 2001), we hypothesized that rvs mutants may have a constitutively activated Slt2p pathway. We used anti-phospho-p42/p44 MAP kinase antibody, which specifically recognizes the phosphorylated (activated) form of Slt2p (Verna et al., 1997; de Nobel et al., 2000), to examine activation of the Slt2p pathway in wild-type and rvs161Δ and rvs167Δ cells. Because we were unable to detect endogenous Slt2p using this antibody (our unpublished data), we transformed cells with a high-copy plasmid encoding HA-tagged Slt2p and immunoprecipitated Slt2p-HA from these cells using anti-HA antibodies, for detection with the anti-phospho-p42/p44 MAP kinase antibody (Madden et al., 1997). We immunoprecipitated Slt2p-HA using anti-HA antiserum from wild-type, rvs161Δ, and rvs167Δ cells grown at 24°C. As a positive control for Slt2p activation, we also activated the Slt2p pathway in a wild-type strain by incubating the cells at 39°C for 2 h (Kamada et al., 1995). No proteins were recognized by the anti-phospho-p42/44 antibody in immunoprecipitates from a wild-type strain with untagged Slt2p (Figure 6A, top). In wild-type cells grown at 24°C, only a small amount of phosphorylated Slt2-HA was detected by the anti-phospho-p42/p44, whereas incubation of cells at 39°C resulted in the accumulation of a larger amount of phosphorylated Slt2p (Figure 6A, top, lanes 2 and 3). Consistent with the hypothesis that Slt2p is constitutively activated in the absence of Rvs161p and Rvs167p, rvs161Δ and rvs167Δ mutants grown at 24°C displayed a higher level of phosphorylated Slt2p than the wild-type strain grown at 24°C (Figure 6A, lanes 4 and 5 respectively). Immunoblot analysis using an anti-HA antibody confirmed that similar amounts of Slt2p were present in every lane (Figure 6A, bottom).
Figure 6.
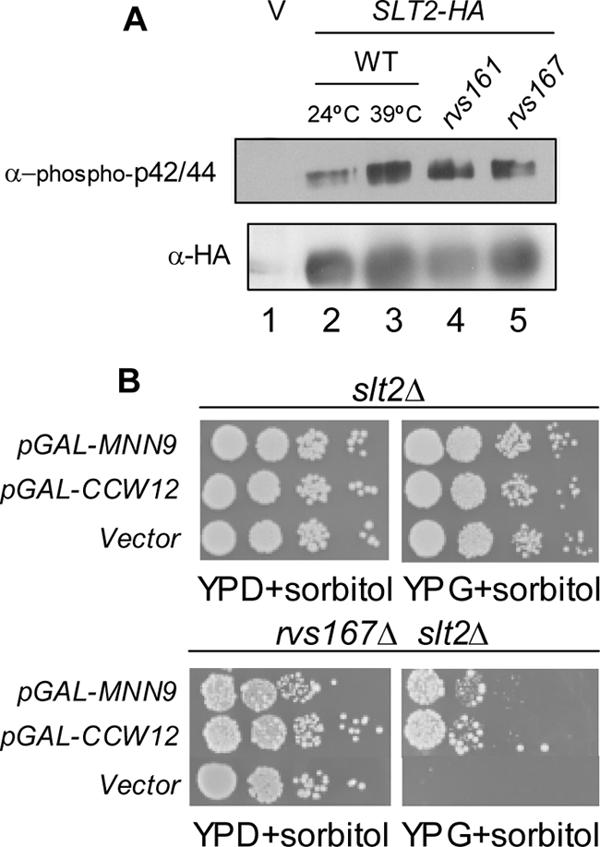
Slt2p is activated in rvs161Δ and rvs167Δ mutants. (A) Immunoblot assay of Slt2p activation. Slt2p-HA was immunoprecipitated with α-HA antibodies from strains harboring either vector (lane 1) or a plasmid containing SLT2-HA (BA1019; lanes 2–5). Lane 2, wild-type strain (BY4741a) grown at 24°C; lane 3, wild-type strain grown at 39°C; lane 4, rvs161Δ strain grown at 24°C; and lane 5, rvs167Δ strain grown at 24°C. Top, immunoblot assays of immunoprecipitated proteins probed with α-phospho-p42/44 MAPK (Thr202/Tyr204) antibodies. Bottom, immunoblot assays of the same samples probed with 12CA5 α-HA antibodies. (B) Suppression of growth defects of an slt2Δ rvs167Δ strain by overexpression of putative downstream targets of the Slt2p pathway. Growth defects of an slt2Δ rvs167Δ strain are suppressed by overproduction of MNN9 and CCW12 but not by vector. slt2Δ or slt2Δ rvs167Δ cells transformed with vector, or plasmids expressing GST-MNN9 or CCW12 under control of the GAL promoter were grown in synthetic dextrose lacking uracil with 1 M sorbitol for plasmid selection. Cells were then assayed by spotting 10-fold serial dilutions on YPD + 1 M sorbitol and on YP + galactose + 1 M sorbitol and incubated for 3 d (slt2Δ) or 6 d (slt2Δ rvs167Δ) at 30°C.
The function of Slt2p in ensuring cellular integrity is mediated in part through the transcriptional induction of genes encoding enzymes involved in cell wall biosynthesis (for review, see Heinisch et al., 1999). Slt2p activates the transcription factors Rlm1p and SBF, a heterodimer composed of Swi4p and Swi6p (Andrews and Herskowitz, 1989), leading to expression of specific subsets of genes (Madden et al., 1997; Watanabe et al., 1997; Jung and Levin, 1999; Baetz et al., 2001). Thus, one possible explanation for the requirement for the Slt2p pathway components in rvs mutants is that genes whose expression is Slt2p-dependent are essential in the absence of Rvs161p and Rvs167p. Consistent with this hypothesis, we had identified two genes whose transcription is known to be dependent on Slt2p, CCW12 and CHS3 (Jung and Levin, 1999; Baetz et al., 2001), in our screen for genes that had synergistic growth defects in combination with deletions of rvs161Δ and rvs167Δ (Figure 2A).
We hypothesized that overproduction of transcriptional targets of Slt2p might rescue the synthetic lethality of the slt2Δ rvs167Δ mutant. The double mutant strain is unable to grow on galactose-containing medium, even in the presence of the osmotic stabilizer sorbitol, but we could keep it alive on glucose-containing medium containing 1 M sorbitol (Figure 6B; Breton et al., 2001). We transformed an slt2Δ strain and an rvs167Δ slt2Δ strain with overexpression plasmids containing the various genes involved in cell wall maintenance that had come out of our synthetic lethality screen and looked for suppression on galactose. Overexpression of two of the genes, MNN9 and CCW12, but not vector alone, could partially suppress the lethality of an rvs167Δ slt2Δ strain (Figure 6B). These data suggest that the essential role of the Slt2p–MAP kinase pathway in the absence of RVS167 may be to activate transcription of genes involved in cell wall maintenance, presumably through the transcription factor Swi4p.
The SH3 Domain of Rvs167p Does Not Bind Actin But Is Required in the Absence of SLA1
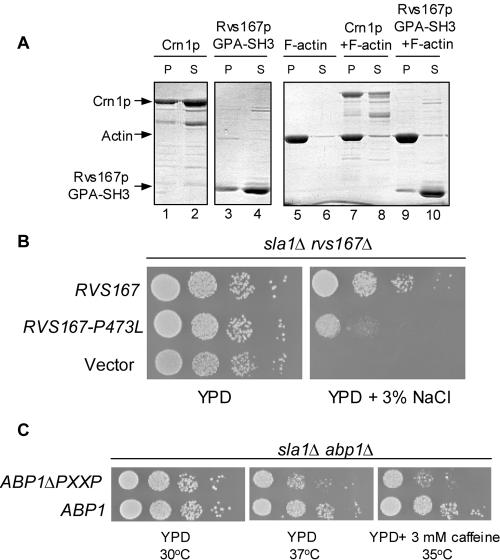
The SH3 domain of Rvs167p interacts with many actin cytoskeleton proteins in two-hybrid studies, including Abp1p, Las17p, and actin (Amberg et al., 1995; Lila and Drubin, 1997; Wendland et al., 1998; Colwill et al., 1999; Bon et al., 2000; Landgraf et al., 2004). A biological role for the SH3 domain remains unclear because it is not required to complement any rvs167Δ defects; the BAR domain of Rvs167p is largely sufficient to rescue all rvs167Δ phenotypes (Colwill et al., 1999). One possible role for the SH3 domain is to bind actin. An interaction between the carboxy-terminal region of Rvs167p and actin has been demonstrated in two-hybrid assays, but it is unclear whether this interaction is direct or whether it is mediated through interactions with other actin-binding proteins (Amberg et al., 1995; Lombardi and Riezman, 2001). To test the carboxy-terminal portion of Rvs167p for binding to actin, we asked whether purified GPA-SH3 domain could bind actin in vitro. GPA-SH3 domain was added to preassembled F-actin, and binding was tested in a high-speed cosedimentation assay. Background levels of GPA-SH3 pelleting (∼20%) were observed in reactions that contained GPA-SH3 alone (Figure 7A, lanes 3 and 4). No increase in GPA-SH3 pelleting was detected in reactions containing F-actin (Figure 7A, lanes 9 and 10). In contrast, despite similar background levels of pelleting, the majority of Crn1p, which was used in this assay as a positive control for F-actin binding (Goode et al., 1999) was detected in the pellet fractions in reactions containing F-actin (Figure 7A, lanes 7 and 8). These data suggest that previous two-hybrid interactions between actin and the carboxy terminus of Rvs167p are indirect (Lila and Drubin, 1997; Lombardi et al., 2001). It is possible, however, that the SH3 domain of Rvs167p has no role in the absence of the BAR domain and depends on interactions between Rvs161p and Rvs167p.
Figure 7.
Role of the SH3 domain of Rvs167p. (A) F-actin binding assay for the GPA-SH3 region of Rvs167. Crn1p (2.5 μM) or His-tagged GPA-SH3 polypeptide (2.5 mM) was incubated with buffer (lanes 1–4) or preassembled actin filaments (5 mM; lanes 7–10). Lanes 5 and 6, F-actin alone. After incubation, the reactions were pelleted at high speed, and the pellet (P) and supernatant (S) fractions were analyzed by SDS-PAGE and Coomassie blue staining. Crn1p (2.5 mM) was included as a positive control for F-actin binding. (B) Biological role of the SH3 domain of Rvs167p. An sla1Δ rvs167Δ strain is complemented by wild-type RVS167 but not by RVS167-P473L or by vector. Ten-fold serial dilutions of BY2805 transformants containing vector, wild-type RVS167 (BA1656) or RVS167-P473L (BA1658) were spotted on YPD or YPD containing salt as indicated. (C) The sla1Δ abp1Δ strain is complemented by ABP1 but not by ABP1ΔPXXP. The sla1Δ abp1Δ cells were kept alive with a wild-type ABP1-URA3 (BA1695) plasmid, transformed with LEU2-based plasmids containing wild-type ABP1 (BA1697) and ABP1ΔPXXP (BA1732) and vector (our unpublished data), and then plated on 5-FOA to select cells that had lost the wild-type ABP1-URA3 plasmid. No colonies containing LEU2-based vector were able to grow on 5-FOA. Survivors were then assayed by spot dilution on YPD or YPD + 3 mM caffeine at the temperatures indicated.
To gain further insight into the biological role of the Rvs167p SH3 domain, we looked for genetic backgrounds in which the SH3 domain of Rvs167p was essential. We tested whether the SH3 domain was required to complement the growth defects of rvs167Δ xxxΔ mutants identified in our SGA analysis. Because both RVS161 and RVS167 were required in the double mutants, it seemed likely that the BAR domain was the essential portion of Rvs167p; however, it was possible that the SH3 domain was important in addition to the BAR domain. We tested the rvs167Δ xxxΔ mutants for complementation using the plasmid that encodes Rvs167p with a mutant SH3 domain (Rvs167p-P473L). We found that RVS167 and RVS167-P473L complemented the synthetic growth defects of most double mutants (our unpublished data), even when we tested for growth on salt-containing medium. However, RVS167-P473L did not complement the synthetic growth defects of the sla1Δ rvs167Δ strain as efficiently as wild-type RVS167 (Figure 7B). In addition, we saw a partial defect in complementation by RVS167-P473L of the end3Δ rvs167Δ strain (our unpublished data). These results show that the SH3 domain of Rvs167p is required for viability in the absence of SLA1 and END3 and provides the first genetic evidence for a role for the Rvs167p SH3 domain.
Our observation that the SH3 domain of Rvs167p is essential in the absence of SLA1 and END3 suggests that the protein that binds to the SH3 domain would also be essential in the absence of these genes. To look for the essential SH3-binding protein, we tested the genes encoding eleven putative SH3-interacting proteins for synthetic lethality with deletion of SLA1 using tetrad analysis: ABP1, ACF2, ACF4, APP1, GYL1, BSP1, GYP5, HUA1, HUA2, YGL060w (YBP2), and YBR108w. We chose these proteins based on their predicted or known interactions with Rvs167p and the presence of SH3 binding motifs (PXXP) in the protein sequences (Pawson and Gish, 1992; Mayer and Eck, 1995; Gavin et al., 2002; Ho et al., 2002; Tong et al., 2002). Some mutants of genes encoding putative Rvs167p SH3 binding proteins, such as Las17p, could not be tested for synthetic lethality because they were extremely slow growing on their own. Ten of 11 of the genes encoding putative Rvs167p SH3-interacting proteins had no synthetic interaction with SLA1 (our unpublished data). Only one of the genes, ABP1, had a synthetic lethal interaction with SLA1, a genetic interaction that has been reported previously (Holtzman et al., 1993). A synthetic interaction between END3 and ABP1 was also detected when double mutants were grown at 37°C (our unpublished data). These data suggested that the interaction between Abp1p and the SH3 domain of Rvs167p might be essential in absence of the cytoskeleton proteins Sla1p and End3p.
To test whether the interaction between Abp1p and the SH3 domain of Rvs167p is essential, we created an allele of ABP1 that encodes Abp1p with a deletion of the putative SH3-binding region (ABP1ΔPXXP). We next asked whether PXXP-dependent interactions with Rvs167p are essential by testing whether ABP1ΔPXXP could complement the growth defect of the sla1Δ abp1Δ cells. We predicted that if the interaction between Rvs167p and Abp1p was essential in the sla1Δ background, a version of Abp1p that no longer contains the putative Rvs167p-SH3 binding region should not complement the growth defects of this strain. Because a sla1Δ abp1Δ strain is dead, we assayed complementation using a plasmid shuffle method (see Materials and Methods). We found that the growth defects of the sla1Δ abp1Δ strain grown at 37°C or grown in the presence of 3 mM caffeine at 35°C were complemented less efficiently by ABP1ΔPXXPI than by wild-type ABP1 (Figure 7C). The end3Δ abp1Δ strain was also complemented less efficiently by ABP1ΔPXXPI than by wild-type ABP1, although the degree of complementation of the ABP1ΔPXXP plasmid in the end3Δ abp1Δ strain was transformant-dependent and variable in penetrance (our unpublished data), possibly because of variability in levels of expression of ABP1ΔPXXP from the plasmid. Thus, under certain growth conditions, the putative Rvs167p-SH3 interaction domain of Abp1p is essential in the absence of Sla1p and End3p. These data support a model in which the interaction between Rvs167p and Abp1p is essential under some physiological conditions.
Membrane Binding Activity of Rvs167p-161p
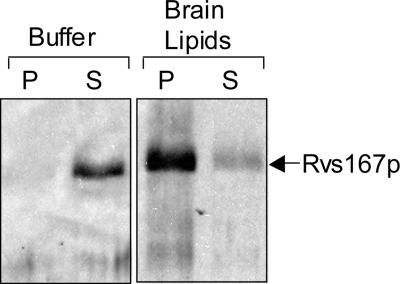
Although Rvs167p GPA-SH3 was not able to bind to F-actin in an actin pelleting assay, we still searched for a biochemical activity associated with Rvs167p. The mammalian homologues of Rvs167p, amphiphysin-1 and amphiphysin-2, and the Drosophila homologue dAmph, all of which act as homodimers, have been shown to bind to lipid membranes and to promote membrane curvature in vitro (Takei et al., 1999; Razzaq et al., 2001; Lee et al., 2002; Peter et al., 2004). We tested whether our purified Rvs161p-167p heterodimer could bind to liposomes using a sedimentation assay as described by Peter et al. (2004). We preincubated Rvs161p-167p with liposomes from brain lipids or with buffer and then subjected the mixture to high-speed centrifugation to pellet the liposomes. We then assayed the supernatant and the pellet for the presence of Rvs167p by Western blot. In this experiment, Rvs167p (and presumably associated Rvs161p) was pelleted in the presence of vesicles from brain lipids but not with buffer alone (Figure 8). Thus, like its homologues, Rvs167p is able to bind to phospholipid membranes.
Figure 8.
Membrane binding by Rvs167p-161p. Sedimentation assay with liposomes made from total bovine brain lipids or buffer alone. After centrifugation, supernatants were removed immediately and pellets were resuspended in a volume of buffer equal to that of the supernatant and then analyzed by PAGE and Western blotting with α-Rvs167p antibodies.
DISCUSSION
Rvs161p and Rvs167p are members of the conserved BAR-domain family of proteins and have established roles in the organization of the actin cytoskeleton and in endocytosis. However, since their identification 14 years ago (Crouzet et al., 1991), the biological function of the Rvs proteins has remained poorly understood. In this work, we have shown that, unlike the Drosophila homologue amphiphysin, which acts as a homodimer, Rvs161p and Rvs167p form an obligate heterodimer. Consistent with this, our genetic experiments reveal that Rvs161p and Rvs167p have a shared role in both growing and mating cells. Our comprehensive analysis of synthetic lethal interactions reveals an important role for the Rvs proteins in actin polarity, cell wall integrity and maintenance, and vesicle trafficking. Finally we show that, like the amphiphysins, the Rvs161p-167p heterodimer is able to bind to liposomes in vitro.
Analysis of Synthetic Lethal Interactions of RVS161 and RVS167
Our synthetic lethal analyses show that 1) RVS161 and RVS167 have synthetic lethal interactions with 49 of 49 of the same genes; 2) the Rvs proteins have roles in cell polarity, cell integrity, cell wall synthesis and maintenance, and vesicle trafficking; and 3) the Slt2–MAP kinase pathway is activated in RVS mutants, and the transcriptional induction of Slt2 targets is required in the absence of RVS genes. Our SGA analyses have significantly expanded the results of previous conventional genetic approaches and tests that have revealed 10 genetic interactions with RVS167 mutants (Lila and Drubin, 1997; Singer-Krüger and Ferro-Novick, 1997; Breton and Aigle, 1998; Breton et al., 2001; Germann et al., 2005); VPS21, SLT2, and SLA1 were identified by both methods. Several previously identified synthetic interactions with rvs167Δ were not uncovered in our screen, in part because some rvs167Δ xxxΔ mutants have only subtle growth defects, for example, kre6Δ rvs167Δ mutants that exhibit growth defects when grown at 37°C (Breton et al., 2001). As well, some mutant strains, such as sla2Δ, srv2Δ, and sac6Δ single mutants are too slow growing to survive the SGA procedure (our unpublished data) and were therefore not present on the final selection plates. We have uncovered 46 new RVS167-requiring genetic backgrounds and have shown that all of these also require RVS161.
Cell Polarity
We identified genetic interactions between RVS161 and RVS167 and genes that encode proteins involved in the regulation of actin filaments (CAP1, CAP2, SLA1, and MYO5) as well as several proteins that have been implicated in the control of the actin assembly through regulation of the Arp2p/3p complex (SLA1, END3, BBC1, and MYO5; Goode and Rodal, 2001). Similarly, the defects in the actin cytoskeleton caused by the decreased yield of folded actin in mutants with defects in the prefoldin complex (pac10, yke2, gim3, gim4, and gim5) may be exacerbated by the loss of Rvs protein expression (Siegers et al., 1999). These data are consistent with a general requirement for Rvs proteins when the actin cytoskeleton is compromised and suggest that loss of more than one function that contributes to actin organization causes inviability. Consistent with this idea, two of the rvs167 double mutants tested (rvs167Δ myo5Δ and rvs167Δ yke2Δ) had a severely depolarized actin cytoskeleton compared with either single mutant (Figure 5A).
Cell Wall Synthesis and Regulation
Results from our SGA analyses show that RVS161 and RVS167 have synthetic lethal interactions with genes that encode machinery for cell wall construction, including 1) components of the Anp1p-Hoc1p-Mnn9p-Mnn10p-Mnn11p mannosyltransferase complex of the Golgi, encoded by MNN2, MNN9, MNN10, and HOC1 (Jungmann and Munro, 1998) (ANP1 had no synthetic interaction with RVS161 or RVS167); 2) CHS3, CHS5, CHS6 CHS7, SKT5, and BNI4, which encode proteins involved in chitin synthase III activity (DeMarini et al., 1997); and 3) KRE1 and KRE11, which encode proteins involved in cell wall β-glucan assembly (Brown et al., 1993). Subtle defects in cell wall morphology have previously been reported for rvs161 and rvs167 mutants (Breton et al., 2001). Thus, rvs mutants likely have a compromised cell wall and are sensitive to additional perturbations in their cell wall caused by deletion of cell wall genes. Consistent with this, Calcofluor White staining revealed that several of the rvs167Δ xxxΔ mutants (rvs167Δ myo5Δ, rvs167Δ sla1Δ, rvs167Δ vps21Δ, and rvs167Δ yke2Δ) had more severe cell wall defects than either of the single mutants (Figure 5B).
The cell wall defects in rvs167Δ and rvs161Δ strains may be a consequence of defects in endocytosis. Actin patches, which are thought to be sites of endocytosis (Kaksonen et al., 2003), localize near sites of secretion, suggesting that they have a role in compensatory endocytosis to recycle plasma membrane and proteins that have been delivered to the plasma membrane during secretion. In mutants with defects in endocytosis, cell wall synthesis may go unchecked and recycling of plasma membrane will not occur. Many mutants with defects in endocytosis (e.g., sla2Δ [Mulholland et al., 1997], sla1Δ [Ayscough et al., 1999], pan1-4 and end3Δ [Tang et al., 2000], and myo3Δ myo5Δ [Goodson et al., 1996]) have defects in cell wall assembly, often having thickened cell walls.
A related category of genes that have synthetic genetic interactions with RVS167 and RVS161 contains genes encoding components of the Sin3p/Rpd3p histone deacetylase complex. PHO23 (Loewith et al., 2001, Gavin et al., 2002), SDS3 (Lechner et al., 2000; Gavin et al., 2002), SAP30 (Zhang et al., 1998; Gavin et al., 2002), DEP1 (Lamping et al., 1994; Gavin et al., 2002), and RXT2 (Gavin et al., 2002) encode proteins associated with the Sin3p-Rpd3p histone deacetylase complex (Kasten et al., 1997). Mutants with defects in components of the Sin3p-Rpd3p histone deacetylase complex have been shown to have defective cell walls (Vannier et al., 2001), presumably because of insufficient or inappropriate expression of cell wall genes.
Cell Integrity MAP Kinase Pathway
A role for the Rvs proteins in cell wall integrity is further supported by our identification of synthetic lethal interactions between rvs mutants and components of the Slt2p–MAP kinase pathway: BCK1, which encodes the MAPK kinase kinase (Lee et al., 1993), and SLT2, which encodes the MAPK. Environmental stress or defects in the cell wall activate the Slt2p pathway leading to the activation of downstream transcription factors and the expression of cell wall genes, which may allow cells to compensate for the cell wall defects (Igual et al., 1996; Madden et al., 1997; Jung and Levin, 1999; Lagorce et al., 2003). We found that deletion of RVS161 or RVS167 causes constitutive activation of the Slt2p MAP kinase and that the growth defects of an slt2Δ rvs167Δ mutant are partially suppressed by overexpression of MNN9 or CCW12, two genes that may be transcriptionally induced by the Slt2p pathway. Thus, rvs161Δ and rvs167Δ mutants require Slt2p activation and the transcriptional induction of at least one Slt2p target. These data also predict that Slt2p target genes may be synthetic lethal with rvs mutants; further analyses of the genes identified in the RVS synthetic lethal screens may allow identification of additional Slt2p transcriptional targets.
Activation of Slt2p results in activation of two transcription factors, Rlm1p (Jung and Levin, 1999) and the heterodimeric transcription factor SBF (composed of Swi4p and Swi6p), leading to expression of downstream targets, including cell wall biosynthesis genes (Madden et al., 1997). We found that RLM1 and SWI6 are not synthetic lethal with rvs167Δ (our unpublished data), suggesting that SWI4 is the only essential downstream effector of Slt2p in the response to cell wall damage in rvs mutants. These data are in agreement with a previous report that revealed a Swi6-independent role for Swi4p and Slt2p in the regulation of a subset of target genes (Baetz et al., 2001). Thus, deletion of RVS161 and RVS167, like other forms of cell wall perturbation, may lead to activation of the Slt2p pathway acting via Swi4p, in response to cell wall damage.
Vesicle Trafficking
Another category of genes showing synthetic interactions with RVS genes encodes proteins involved in vesicle trafficking. We have found that the rvs delete strains have synthetic growth defects in combination with two genes involved in vesicle trafficking from endoplasmic reticulum (ER) to Golgi, sec22Δ and rud3Δ (Newman and Ferro-Novick, 1990; Kim, 2003), suggesting that the Rvs proteins may be required for proper ER-to-Golgi trafficking. Additional evidence to support a role for Rvs167p in ER-to-Golgi vesicle trafficking comes from screens for proteins that interact with the SH3 domain of Rvs167p: the Rvs167p SH3 domain has been shown to bind to at least one protein involved in vesicle trafficking from ER to Golgi: Gyp5p (Bon et al., 2000; Ho et al., 2002; Chesneau et al., 2004; Friesen et al., 2005) as well as the Gyp5p-interacting protein Gyl1p (Chesneau et al., 2004; Friesen et al., 2005). In addition, the finding that RVS167 and RVS161 have synthetic genetic interactions with VPS21, a gene involved in the delivery of vacuolar and endocytosed proteins to the vacuole (Gerrard et al., 2000; interaction with RVS167 reported previously by Singer-Krüger and Ferro-Novick, 1997) suggests a role for Rvs161p-167p in multiple vesicle-mediated functions.
The classes of genes that have synthetic genetic interactions with RVS161 and RVS167, genes required for cell wall synthesis and maintenance and vesicle trafficking, may be connected through a need for proper vesicle formation or fusion. The recent finding that the Rvs homologue amphiphysin binds to liposomes and promotes membrane curvature in vitro (Takei et al., 1999; Razzaq et al., 2001; Lee et al., 2002; Peter et al., 2004), and our observation that purified Rvs167p-161p binds to liposomes in vitro (Figure 8) is consistent with a role for Rvs161p-167p in vesicle biogenesis or fusion. It is not clear whether a role for the Rvs proteins in vesicle biology would be distinct from their role in the actin cytoskeleton and endocytosis.
Both Rvs161p and Rvs167p Have a Role in Mating
In mating assays with a subset of the genes identified in our mating screen, we found that, like rvs161Δ, rvs167Δ has synthetic mating defects in combination with deletion of PRM9 and FIG2, but unlike rvs161Δ, it has no defects with FUS1 and AXL1. Our results are in agreement with a previous report, which showed no defects in mating of an rvs167Δ fus1Δ mutant (Brizzio et al., 1998). However, we found a requirement for efficient mating for both RVS161 and RVS167 in certain genetic backgrounds, revealing a probable role for the Rvs161p-Rvs167p heterodimer during mating. Recently, others have also reported a defect in mating in rvs167Δ cells (Talarek et al., 2004). Consistent with a role for Rvs167p in mating, we find that Rvs161p requires Rvs167p for stability during mating (Figure 3, A and B), and Rvs167p becomes hyperphosphorylated in cells exposed to pheromone (Figure 3B; Lee et al., 1998; Friesen et al., 2003). In addition, Rvs167p localizes to the shmoo tip and the cell fusion zone in mated cells and rvs167Δ mutants have delayed cell fusion in prezygotes (Balguerie et al., 1999; Breton et al., 2001).
Our data suggest that the Rvs proteins have two roles in mating. The first role is seen for Rvs161p, is essential in the absence of Fus1p, and is Rvs167p independent (Brizzio et al., 1998; Figure 4A). The second role, for which we provide evidence in this manuscript, requires both Rvs161p and Rvs167p and is essential in the absence of Prm9p and Fig2p (Figure 4A). The defect in mating in the rvs167Δ strain was not suppressed by overexpressing RVS161 (Figure 4C), consistent with a requirement for both Rvs proteins. This role in mating does not seem to be a role for endocytosis per se, because two other genes required for endocytosis, SLA1 and END3, are not required for mating in the absence of Prm9p and Fig2p (Figure 4C).
A Role for the SH3 Domain of Rvs167p
Although the SH3 domain of Rvs167p is conserved among amphiphysins, its biological function in S. cerevisiae remains unknown. We found that a version of Rvs167p with a defective SH3 domain was able to complement almost all of our rvs167Δ xxxΔ mutants, indicating that the SH3 domain was not important for Rvs167p activity under standard growth conditions. In contrast, we found that the SH3 domain was important in the absence of SLA1 and END3 under certain unfavorable growth conditions (on YPD + 3% NaCl; Figure 7B) and that this requirement likely requires a functional interaction between the SH3 domain of Rvs167p and Abp1p. We found previously that the phosphorylation sites of Rvs167p are important for growth on salt-containing medium in the absence of SLA1 or END3 and that phosphorylation of Rvs167p affects binding to the SH3 domain of some ligands (Friesen et al., 2003). These data are consistent with a model in which the interaction with Rvs167p and Abp1p (and the regulation of this interaction by phosphorylation) is essential in a sla1Δ or an end3Δ background. Sla1p and End3p form a complex with a third (essential) protein Pan1p (Tang et al., 2000), which activates the Arp2p/3p complex to stimulate actin polymerization (Duncan et al., 2001). Abp1p has also been found to stimulate the Arp2p/3p complex (Goode et al., 2001), so one possibility is that in the absence of the Sla1p-End3p-Pan1p Arp2p/3p-activator complex, it becomes essential for Rvs167p to bind to the alternate Arp2p/3p activator Abp1p.
In summary, we have demonstrated that the yeast amphiphysin homologues Rvs161p and Rvs167p function together in vegetative cells and that the interaction with Rvs161p is the main interaction for Rvs167p. We have shown that Rvs167p plays a role in the mating response that is not a consequence of its stabilizing Rvs161p. We have presented evidence that the interaction between the prolinerich region of Abp1p and the SH3 domain of Rvs167p may be important for actin cytoskeleton organization. The results of our study identify an important link between Rvs161p and Rvs167p and the organization of the actin cytoskeleton, cell wall, and vesicle trafficking during vegetative growth and reveal a requirement for the Rvs161p-167p heterodimer during the mating response. These finding implicate heterodimerization between other amphiphysins as an important interaction for the function of these molecules in various cellular processes.
Acknowledgments
We thank Wei Ye and Trinh Hoac for providing technical assistance. We thank Richelle Sopko and Jennifer Haynes for plasmids and for help with microscopy. We are grateful to Jon Millman for helpful discussions. We thank Bianca Garcia and Alan Davidson for constructing pRS315-ABP1. This work was supported by an operating grant from the National Cancer Institute of Canada with funds from the Canadian Cancer Society. Our SGA laboratories are supported by a Collaborative Genomics Special Project grant from the Canadian Institutes of Health Research and by funds from Genome Canada through the Ontario Genomics Institute.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–06–0476) on January 4, 2006.
References
- Amberg, D. C., Basart, E., and Botstein, D. (1995). Defining protein interactions with yeast actin in vivo. Nat. Struct. Biol. 2, 28–35. [DOI] [PubMed] [Google Scholar]
- Andrews, B. J., and Herskowitz, I. (1989). Identification of a DNA binding factor involved in cell cycle-control of the yeast HO gene. Cell 57, 21–29. [DOI] [PubMed] [Google Scholar]
- Ayscough, K. A., and Drubin, D. G. (1996). Actin: general principles from studies with yeast. Annu. Rev. Cell Dev. Biol. 12, 129–160. [DOI] [PubMed] [Google Scholar]
- Ayscough, K. R., Eby, J. J., Lila, T., Dewar, H., Kozminski, K. G., and Drubin, D. G. (1999). Sla1p is a functionally modular component of the yeast cortical actin cytoskeleton required for correct localization of both Rho1p-GTPase and Sla2p, a protein with talin homology. Mol. Biol. Cell 10, 1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetz, K., Moffat, J., Haynes, J., Chang, M., and Andrews, B. (2001). Transcriptional coregulation by the cell integrity mitogen-activated protein kinase Slt2 and the cell cycle regulator Swi4. Mol. Cell. Biol. 21, 6515–6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balguerie, A., Bagnat, M., Bonneu, M., Aigle, M., and Breton, A. M. (2002). Rvs161p and sphingolipids are required for actin repolarization following salt stress. Eukaryot. Cell 1, 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balguerie, A., Sivadon, P., Bonneu, M., and Aigle, M. (1999). Rvs167p, the budding yeast homolog of amphiphysin, colocalizes with actin patches. J. Cell Sci. 112, 2529–2537. [DOI] [PubMed] [Google Scholar]
- Bauer, F., Urdaci, M., Aigle, M., and Crouzet, M. (1993). Alteration of a yeast SH3 protein leads to conditional viability and defects in cytoskeletal and budding patterns. Mol. Cell. Biol. 13, 5070–5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, A., and Pringle, J. R. (1991). Use of a screen for synthetic lethal and multicopy suppressor mutants to identify two new genes involved in morphogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 11, 1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bon, E., Recordon-Navarro, P., Durrens, P., Iwase, M., Toh-E. A., and Aigle, M. (2000). A network of proteins around Rvs167p and Rvs161p, two proteins related to the yeast actin cytoskeleton. Yeast 13, 1229–1241. [DOI] [PubMed] [Google Scholar]
- Brachmann, C. B., Davies, A., Cost, G. J., Caputo, E., Li, J., Hieter, P., and Boeke, J. D. (1998). Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14, 115–132. [DOI] [PubMed] [Google Scholar]
- Breton, A., and Aigle, M. (1998). Genetic and functional relationship between Rvsp, myosin and actin in Saccharomyces cerevisiae. Curr. Genet. 34, 280–286. [DOI] [PubMed] [Google Scholar]
- Breton, A. M., Schaeffer, .J., and Aigle, M. (2001). The yeast Rvs161 and Rvs167 proteins are involved in secretory vesicles targeting the plasma membrane and in cell integrity. Yeast 18, 1053–1068. [DOI] [PubMed] [Google Scholar]
- Brizzio, V., Gammie, A. E., and Rose, M. D. (1998). Rvs161p interacts with Fus2p to promote cell fusion in Saccharomyces cerevisiae. J. Cell Biol. 141, 567–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J. L., Kossaczka, Z., Jiang, B., and Bussey, H. (1993). A mutational analysis of killer toxin resistance in Saccharomyces cerevisiae identifies new genes involved in cell wall (1→6)-beta-glucan synthesis. Genetics 133, 837–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesneau, L., Dupre, S., Burdina, A., Roger, J., LePanse, S., Jacquet, M., and Cuif, M.-H. (2004). Gyp5p and Gyl1p are involved in the control of polarized exocytosis in budding yeast. J. Cell Sci. 117, 4757–4767. [DOI] [PubMed] [Google Scholar]
- Colwill, K., Field, D., Moore, L., Friesen, J., and Andrews, B. (1999). In vivo analysis of the domains of yeast Rvs167p suggests Rvs167p function is mediated through multiple protein interactions. Genetics 152, 881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet, M., Urdaci, M., Dulau, L., and Aigle, M. (1991). Yeast mutant affected for viability upon nutrient starvation: characterization and cloning of the RVS161 gene. Yeast 7, 727–743. [DOI] [PubMed] [Google Scholar]
- DeMarini, D. J., Adams, A. E., Fares, H., De Virgilio, C., Valle, G., Chuang, J. S., and Pringle, J. R. (1997). A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J. Cell Biol. 139, 75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nobel, H., Ruiz, C., Martin, H., Morris, W., Brul, S., Molina, M., and Klis, F. M. (2000). Cell wall perturbation in yeast results in dual phosphorylation of the Slt2/Mpk1 MAP kinase and in an Slt2-mediated increase in FKS2-lacZ expression, glucanase resistance and thermotolerance. Microbiology 146, 2121–2132. [DOI] [PubMed] [Google Scholar]
- Desfarges, L., Durrens, P., Juguelin, H., Cassagne, C., Bonneu, M., and Aigle, M. (1993). Yeast mutants affected in viability upon starvation have a modified phospholipid composition. Yeast 9, 267–277. [DOI] [PubMed] [Google Scholar]
- Drees, B. L., et al. (2001). A protein interaction map for cell polarity development. J. Cell Biol. 154, 549–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, M. C., Cope, M. J., Goode, B. L., Wendland, B., and Drubin, D. G. (2001). Yeast Eps15-like endocytic protein, Pan1p, activates the Arp2/3 complex. Nat. Cell Biol. 3, 687–690. [DOI] [PubMed] [Google Scholar]
- Elia, L., and Marsh, L. (1998). A role for a protease in morphogenic responses during yeast cell fusion. J. Cell Biol. 142, 1473–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen, H., Colwill, K., Roberts, K., Schub, O., and Andrews, B. (2005). Interaction of the Saccharomyces cerevisiae cortical actin patch protein Rvs167p with proteins involved in ER to Golgi vesicle trafficking. Genetics 170, 555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen, H., Murphy, K., Breitkreutz, A., Tyers, M., and Andrews, B. (2003). Regulation of the yeast amphiphysin homologue Rvs167p by phosphorylation. Mol. Biol. Cell 7, 3027–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, A., Oka, C., Arikawa, Y., Katagai, T., Tonouchi, A., Kuhara, S., and Misumi, Y. (1994). A yeast gene necessary for bud-site selection encodes a protein similar to insulin-degrading enzymes. Nature 372, 567–570. [DOI] [PubMed] [Google Scholar]
- Gavin, A. C., et al. (2002). Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415, 141–147. [DOI] [PubMed] [Google Scholar]
- Geli, M. I., and Riezman, H. (1996). Role of type I myosins in receptor-mediated endocytosis in yeast. Science 272, 533–535. [DOI] [PubMed] [Google Scholar]
- Gerrard, S. R., Bryant, N. J., and Stevens, T. H. (2000). VPS21 controls entry of endocytosed and biosynthetic proteins into the yeast prevacuolar compartment. Mol. Biol. Cell 11, 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann, M., Swain, E., Bergman, L., and Nickels, J. T., Jr. (2005). Characterizing the sphingolipid signaling pathway that remediates defects associated with loss of the yeast amphiphysin-like orthologs, Rvs161p and Rvs167p. J. Biol. Chem. 280, 4270–4278. [DOI] [PubMed] [Google Scholar]
- Goode, B. L., and Rodal, A. A. (2001). Modular complexes that regulate actin assembly in budding yeast. Curr. Opin. Microbiol. 4, 703–712. [DOI] [PubMed] [Google Scholar]
- Goode, B. L., Rodal, A. A., Barnes, G., and Drubin, D. G. (2001). Activation of the Arp2/3 complex by the actin filament binding protein Abp1p. J. Cell Biol. 153, 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode, B. L., Wong, J. J., Butty, A.-C., Peter, M., McCormack, A., Yates, J. R., Drubin, D. G., and Barnes, G. (1999). Coronin promotes the rapid assembly and cross-linking of actin filaments and may link the actin and microtubule cytoskeletons in yeast. J. Cell Biol. 144, 83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson, H. V., Anderson, B. L., Warrick, H. M., Pon, L. A., and Spudich, J. A. (1996). Synthetic lethality screen identifies a novel yeast myosin I gene (MYO5): myosin I proteins are required for polarization of the actin cytoskeleton. J. Cell Biol. 133, 1277–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, C., and Fink, G. R. (1991). Guide to Yeast Genetics and Molecular Biology, San Diego: Academic.
- Heiman, M. G., and Walter, P. (2000). Prm1p, a pheromone-regulated multi-spanning membrane protein, facilitates plasma membrane fusion during yeast mating. J. Cell Biol. 151, 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinisch, J., Lorberg, A., Schmitz, H.-P., and Jacoby, J. (1999). The protein kinase C (PKC)-mediated MAP kinase pathway involved in the maintenance of cellular integrity in Saccharomyces cerevisiae. Mol. Microbiol. 32, 671–680. [DOI] [PubMed] [Google Scholar]
- Ho, Y., et al. (2002). Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415, 180–183. [DOI] [PubMed] [Google Scholar]
- Holtzman, D. A., Yang, S., and Drubin, D. (1993). Synthetic-lethal interactions identify two novel genes, SLA1 and SLA2, that control membrane cytoskeleton assembly in Saccharomyces cerevisiae. J. Cell Biol. 122, 635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries, C. L., Balcer, H. I., D'Agostino, J. L., Winsor, B., Drubin, D. G., Barnes, G., Andrews, B. J., and Goode, B. L. (2002). Direct regulation of Arp2/3 complex activity and function by the actin binding protein coronin. J. Cell Biol. 159, 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igual, J. C., Johnson, A. L., and Johnston, L. H. (1996). Coordinated regulation of gene expression by the cell cycle transcription factor Swi4 and the PKC MAP kinase pathway for yeast cell integrity. EMBO J. 15, 5001–5013. [PMC free article] [PubMed] [Google Scholar]
- Ito, T., Chiba, T., Ozawa, R., Yoshida, M., Hattori, M., and Sakaki, Y. (2001). A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA 98, 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, U. S., and Levin, D. E. (1999). Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signaling pathway. Mol. Microbiol. 34, 1049–1057. [DOI] [PubMed] [Google Scholar]
- Jungmann, J., and Munro, S. (1998). Multi-protein complexes in the cis Golgi of Saccharomyces cerevisiae with alpha-1,6-mannosyltransferase activity. EMBO J. 17, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen, M., Sun, Y., and Drubin, D. G. (2003). A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell 115, 475–487. [DOI] [PubMed] [Google Scholar]
- Kamada, Y., Jung, U. S., Piotrowski, J., and Levin, D. E. (1995). The PKC-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9, 1559–1571. [DOI] [PubMed] [Google Scholar]
- Kasten, M. M., Dorland, S., and Stillman, D. J. (1997). A large protein complex containing the yeast Sin3p and Rpd3p transcriptional regulators. Mol. Cell. Biol. 17, 4852–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. W. (2003). Characterization of Grp1p, a novel cis-Golgi matrix protein. Biochem. Biophys. Res. Commun. 303, 370–378. [DOI] [PubMed] [Google Scholar]
- Lagorce, A., Hauser, N. C., Labourdette, D., Rodriguez, C., Martin-Yken, H., Arroyo, J., Hoheisel, J. D., and Francois, J. (2003). Genome-wide analysis of the response to cell wall mutations in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 278, 20345–20357. [DOI] [PubMed] [Google Scholar]
- Lamping, E., Luckl, J., Paltauf, F., Henry, S. A., and Kohlwein, S. D. (1994). Isolation and characterization of a mutant of Saccharomyces cerevisiae with pleiotropic deficiencies in transcriptional activation and repression. Genetics 137, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf, C., Panni, S., Montecchi-Palazzi, L., Castagnoli, L., Schneider-Mergener, J., Volkmer-Engert, R., and Cesareni, G. (2004). Protein interaction networks by proteome peptide scanning. PLoS Biol. 2, E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner, T., Carrozza, M. J., Yu, Y., Grant, P. A., Eberharter, A., Vannier, D., Brosch, G., Stillman, D. J., Shore, D., and Workman, J. L. (2000). Sds3 (suppressor of defective silencing 3) is an integral component of the yeast Sin3-Rpd3 histone deacetylase complex and is required for histone deacetylase activity. J. Biol. Chem. 275, 40961–40966. [DOI] [PubMed] [Google Scholar]
- Lee, E., Marcucci, M., Daniell, L., Pypaert, M., Weisz, O. A., Ochoa, G. C., Farsad, K., Wenk, M. R., and De Camilli, P. (2002). Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science 297, 1193–1196. [DOI] [PubMed] [Google Scholar]
- Lee, J., Colwill, K., Aneliunas, V., Tennyson, C., Moore, L., Ho, Y., and Andrews, B. (1998). Interaction of yeast Rvs167 and Pho85 cyclin-dependent kinase complexes may link the cell cycle to the actin cytoskeleton. Curr. Biol. 8, 1310–1321. [DOI] [PubMed] [Google Scholar]
- Lee, K. S., Irie, K., Gotoh, Y., Watanabe, Y., Araki, H., Nishida, E., Matsumoto, K., and Levin, D. E. (1993). A yeast mitogen-activated protein kinase homolog (Mpk1p) mediates signaling by PKC. Mol. Cell. Biol. 13, 3067–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M. C., and Schekman, R. (2004). Cell biology. BAR domains go on a bender. Science 303, 479–480. [DOI] [PubMed] [Google Scholar]
- Letunic, I., Copley, R. R., Schmidt, S., Ciccarelli, F. D., Doerks, T., Schultz, J., Ponting, C. P., and Bork, P. (2004). SMART 4. 0, towards genomic data integration. Nucleic Acids Res. 32, D142–D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lila, T., and Drubin, D. (1997). Evidence for physical and functional interactions among two Saccharomyces cerevisiae SH3 domain proteins, an adenylyl cyclase-associated protein and the actin cytoskeleton. Mol. Biol. Cell 8, 367–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewith, R., Smith, J. S., Meijer, M., Williams, T. J., Bachman, N., Boeke, J. D., and Young, D. (2001). Pho23 is associated with the Rpd3 histone deacetylase and is required for its normal function in regulation of gene expression and silencing in Saccharomyces cerevisiae. J. Biol. Chem. 276, 24068–24074. [DOI] [PubMed] [Google Scholar]
- Lombardi, R., and Riezman, H. (2001). Rvs161p and Rvs167p, the two yeast amphiphysin homologs, function together in vivo. J. Biol. Chem. 276, 6016–6022. [DOI] [PubMed] [Google Scholar]
- Longtine, M. S., McKenzie, A., DeMarini, D. J., Shah, N. G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J. R. (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961. [DOI] [PubMed] [Google Scholar]
- Madden, K., Sheu, Y.-J., Baetz, K., Andrews, B., and Snyder, M. (1997). SBF cell cycle regulator as a target of the yeast PKC-MAP kinase pathway. Science 275, 1781–1784. [DOI] [PubMed] [Google Scholar]
- Maxwell, K. L., Mittermaier, A. K., Forman-Kay, J. D., and Davidson, A. R. (1999). A simple in vivo assay for increased protein solubility. Protein Sci. 8, 1908–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, B. J., and Eck, M. J. (1995). SH3 domains. Minding your p's and q's. Curr. Biol. 1, 364–367. [DOI] [PubMed] [Google Scholar]
- Measday, V., Moore, L., Ogas, J., Tyers, M., and Andrews, B. (1994). The PCL2 (ORFD)-PHO85 cyclin-dependent kinase complex: a cell cycle regulator in yeast. Science 266, 1391–1395. [DOI] [PubMed] [Google Scholar]
- Mulholland, J., Wesp, A., Riezman, H., and Botstein, D. (1997). Yeast actin cytoskeleton mutants accumulate a new class of Golgi-derived secretary vesicle. Mol. Biol. Cell 8, 1481–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro, P., Durrens, P., and Aigle, M. (1997). Protein-protein interaction between the RVS161 and RVS167 gene products of Saccharomyces cerevisiae. Biochim. Biophys. Acta 1343, 187–192. [DOI] [PubMed] [Google Scholar]
- Newman, A. P., and Ferro-Novick, S. (1990). Defining components required for transport from the ER to the Golgi complex in yeast. Bioessays 12, 485–491. [DOI] [PubMed] [Google Scholar]
- Novick, P., and Botstein, D. (1985). Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell 40, 405–416. [DOI] [PubMed] [Google Scholar]
- Pawson, T., and Gish, G. (1992). SH2 and SH3 domains: from structure to function. Cell 71, 359–362. [DOI] [PubMed] [Google Scholar]
- Pawson, T., and Scott, J. D. (1997). Signaling through scaffold, anchoring, and adaptor proteins. Science 278, 2075–2080. [DOI] [PubMed] [Google Scholar]
- Peter, B. J., Kent, H. M., Mills, I. G., Vallis, Y., Butler, P. J., Evans, P. R., and McMahon, H. T. (2004). BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303, 495–499. [DOI] [PubMed] [Google Scholar]
- Raths, S., Rohrer, J., Crausaz, F., and Riezman, H. (1993). end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J. Cell Biol. 120, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaq, A., Robinson, I. M., McMahon, H. T., Skepper, J. N., Su, Y., Zelhof, A. C., Jackson, A. P., Gay, N. J., and O'Kane, C. J. (2001). Amphiphysin is necessary for organization of the excitation-contraction coupling machinery of muscles, but not for synaptic vesicle endocytosis in Drosophila. Genes Dev. 15, 2967–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamuro, D., Elliott, K. J., Wechsler-Reya, R., and Prendergast, G. C. (1996). BIN1 is a novel MYC-interacting protein with features of a tumour suppressor. Nat. Genet. 14, 69–77. [DOI] [PubMed] [Google Scholar]
- Sherman, F. (1991). Getting started with yeast. Methods Enzymol. 194, 3–21. [DOI] [PubMed] [Google Scholar]
- Siegers, K., Waldmann, T., Leroux, M. R., Grein, K., Shevchenko, A., Schiebel, E., and Hartl, F. U. (1999). Compartmentation of protein folding in vivo: sequestration of non-native polypeptide by the chaperonin-GimC system. EMBO J. 18, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R. S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Krüger, B., and Ferro-Novick, S. (1997). Use of a synthetic lethal screen to identify yeast mutants impaired in endocytosis, vacuolar protein sorting and the organization of the cytoskeleton. Eur. J. Cell Biol. 74, 365–375. [PubMed] [Google Scholar]
- Singer-Krüger, B., Stenmark, H., Dusterhoft, A., Philippsen, P., Yoo, J. S., Gallwitz, D., and Zerial, M. (1994). Role of three rab5-like GTPases, Ypt51p, Ypt52p, and Ypt53p, in the endocytic and vacuolar protein sorting pathways of yeast. J. Cell Biol. 125, 283–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivadon, P., Bauer, F., Aigle, M., and Crouzet, M. (1995). Actin cytoskeleton and budding patterns are altered in the yeast rvs161 mutant: the Rvs161 protein shares common domains with the brain protein amphiphysin. Mol. Gen. Genet. 246, 485–495. [DOI] [PubMed] [Google Scholar]
- Sivadon, P., Crouzet, M., and Aigle, M. (1997). Functional assessment of the yeast Rvs161 and Rvs167 protein domains. FEBS Lett. 417, 21–27. [DOI] [PubMed] [Google Scholar]
- Takei, K., Slepnev, V. I., Haucke, V., and De Camilli, P. (1999). Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell Biol. 1, 33–39. [DOI] [PubMed] [Google Scholar]
- Talarek, N., Balguerie, A., Aigle, M., and Durrens, P. (2005). A novel link between a Rab GTPase and Rvs proteins: the yeast amphiphysin homologues. Cell Biochem. Funct. 23, 253–266. [DOI] [PubMed] [Google Scholar]
- Tang, H. Y., Xu, J., and Cai, M. (2000). Pan1p, End3p, and S1a1p, three yeast proteins required for normal cortical actin cytoskeleton organization, associate with each other and play essential roles in cell wall morphogenesis. Mol. Cell. Biol. 20, 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennyson, C., Lee, J., and Andrews, B. (1998). A role for the Pcl9-Pho85 cyclin-Cdk complex at the M/G1 boundary in Saccharomyces cerevisiae. Mol. Microbiol. 28, 69–79. [DOI] [PubMed] [Google Scholar]
- Tong, A. H., et al. (2004). Global mapping of the yeast genetic interaction network. Science 303, 808–813. [DOI] [PubMed] [Google Scholar]
- Tong, A.H.Y., et al. (2001). Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294, 2364–2368. [DOI] [PubMed] [Google Scholar]
- Tong, A.H.Y., et al. (2002). A combined experimental and computational strategy to define protein interaction networks for peptide recognition modules. Science 5553, 321–324. [DOI] [PubMed] [Google Scholar]
- Uetz, P., et al. (2000). A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403, 623–627. [DOI] [PubMed] [Google Scholar]
- Vannier, D., Damay, P., and Shore, D. (2001). A role for Sds3p, a component of the Rpd3p/Sin3p deacetylase complex, in maintaining cellular integrity in Saccharomyces cerevisiae. Mol. Genet. Genomics 265, 560–568. [DOI] [PubMed] [Google Scholar]
- Verna, J., Lodder, A., Lee, K., Vagts, A., and Ballester, R. (1997). A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94, 13804–13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, D. T., Andrews, P. D., Gourlay, C. W., and Ayscough, K. R. (2002). Sla1p couples the yeast endocytic machinery to proteins regulating actin dynamics. J. Cell Sci. 115, 1703–1715. [DOI] [PubMed] [Google Scholar]
- Watanabe, Y., Takaesu, G., Hagiwara, M., Irie, K., and Matsumoto, K. (1997). Characterization of a serum response factor-like protein in Saccharomyces cerevisiae, Rlm1, which has transcriptional activity regulated by the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17, 2615–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland, B., Emr, S. D., and Reizman, H. (1998). Protein traffic in the yeast endocytic and vacuolar protein sorting pathways. Curr. Opin. Cell Biol. 10, 513–522. [DOI] [PubMed] [Google Scholar]
- Wigge, P., Kohler, K., Vallis, Y., Doyle, C. A., Owen, D., Hunt, S., and McMahon, H. (1997). Amphiphysin heterodimers: potential role in clathrin-mediated endocytosis. Mol. Biol. Cell 8, 2003–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, I. A., Niman, H. L., Houghten, R. A., Cherenson, A. R., Connolly, M. L., and Lerner, R. A. (1984). The structure of an antigenic determinant in a protein. Cell 37, 767–778. [DOI] [PubMed] [Google Scholar]
- Winzeler, E. A., et al. (1999). Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285, 901–906. [DOI] [PubMed] [Google Scholar]
- Yang, S., Ayscough, K. R., and Drubin, D. G. (1997). A role for the actin cytoskeleton of S. cerevisiae in bipolar bud-site selection. J. Cell Biol. 136, 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzov, P., Mazzoni, C., and Mann, C. (1996). The SLT2 (MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO J. 15, 83–91. [PMC free article] [PubMed] [Google Scholar]
- Zhang, B., and Zelhof, A. C. (2002). Amphiphysins: raising the BAR for synaptic vesicle recycling and membrane dynamics. Bin-Amphiphysin-Rvsp. Traffic 3, 452–460. [DOI] [PubMed] [Google Scholar]
- Zhang, M., Bennett, D., and Erdman, S. E. (2002). Maintenance of mating cell integrity requires the adhesin Fig2p. Eukaryot. Cell 1, 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Sun, Z. W., Iratni, R., Erdjument-Bromage, H., Tempst, P., Hampsey, M., and Reinberg, D. (1998). SAP30, a novel protein conserved between human and yeast, is a component of a histone deacetylase complex. Mol. Cell 1, 1021–1031. [DOI] [PubMed] [Google Scholar]
- Zhu, H., Klemic, J. F., Chang, S., Bertone, P., Casamayor, A., Klemic, K. G., Smith, D., Gerstein, M., Reed, M. A., and Snyder, M. (2000). Analysis of yeast protein kinases using protein chips. Nat. Genet. 26, 283–289. [DOI] [PubMed] [Google Scholar]