Abstract
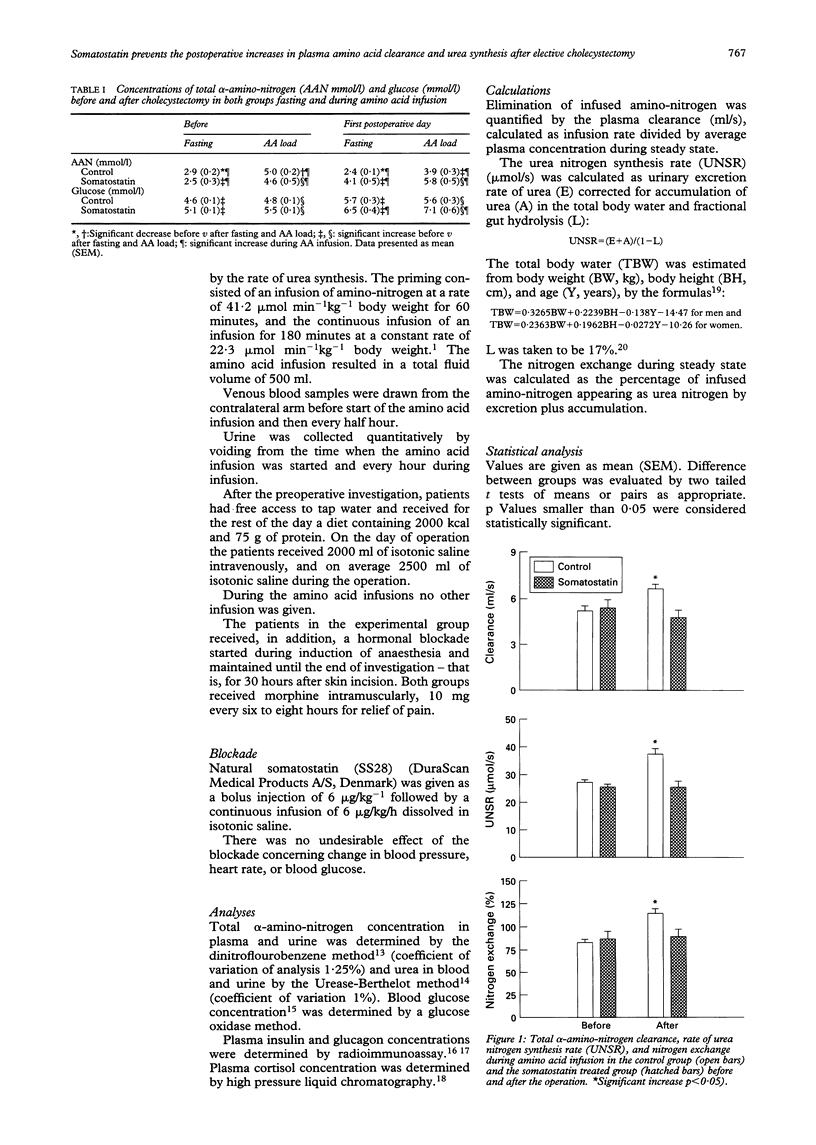
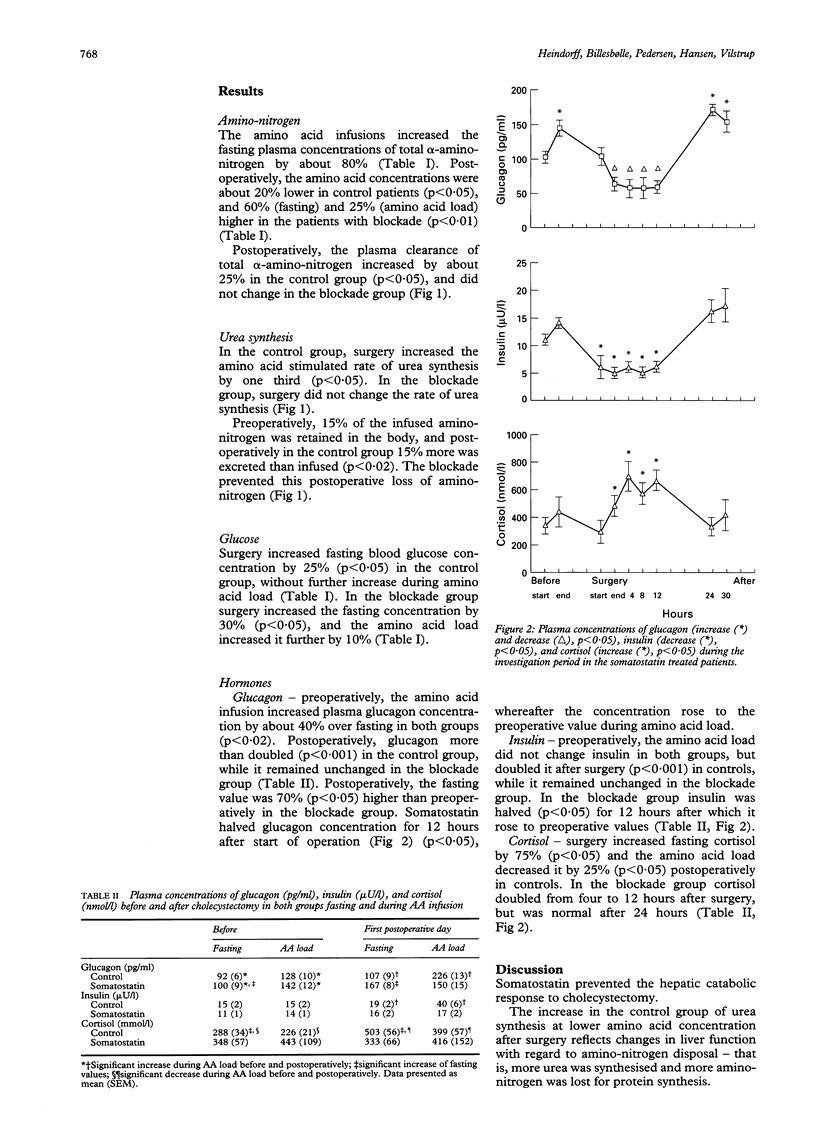
The importance of glucagon on postoperative changes in hepatic amino-nitrogen conversion were investigated in six patients undergoing elective cholecystectomy for uncomplicated gall stones. Patients were given infusions of somatostatin (bolus of 6 micrograms/kg followed by continuous infusion of 6 micrograms/kg/h) from induction of anaesthesia to the end of investigation, the first postoperative day (30 hours). Controls were 16 patients undergoing the same procedures omitting the somatostatin infusion. In all patients blood concentration and plasma clearance of total alpha-amino-nitrogen, and amino acid stimulated rate of urea synthesis were measured. Elective cholecystectomy decreased blood alpha-amino-nitrogen concentration from mean (SEM) 2.9 (0.2) to 2.4 (0.1) mmol/l (p < 0.05), increased the clearance of total alpha-amino-nitrogen from 5.2 (0.3) to 6.6 (0.3) ml/s (p < 0.05), and increased the rate of amino acid stimulated urea synthesis from 27 (1) to 37 (2) mumol/s (p < 0.05) pointing to increased hepatic removal of amino-nitrogen at expense of plasma amino-nitrogen. Infusion of somatostatin prevented increase of glucagon for 24 hours after surgery, and prevented the negative changes in postoperative nitrogen homeostasis resulting from the postoperative changes in hepatic nitrogen conversion, suggesting glucagon as mediator. The exact mechanism remains in doubt, however, because of the multiple effects of somatostatin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian T. E., Barnes A. J., Long R. G., O'Shaughnessy D. J., Brown M. R., Rivier J., Vale W., Blackburn A. M., Bloom S. R. The effect of somatostatin analogs on secretion of growth, pancreatic, and gastrointestinal hormones in man. J Clin Endocrinol Metab. 1981 Oct;53(4):675–681. doi: 10.1210/jcem-53-4-675. [DOI] [PubMed] [Google Scholar]
- Asoh T., Shirasaka C., Uchida I., Tsuji H. Effects of indomethacin on endocrine responses and nitrogen loss after surgery. Ann Surg. 1987 Dec;206(6):770–776. doi: 10.1097/00000658-198712000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton R. N., Passingham B. J. Effect of binding to plasma proteins on the interpretation of plasma cortisol concentrations after accidental injury. Clin Sci (Lond) 1981 Oct;61(4):399–405. doi: 10.1042/cs0610399. [DOI] [PubMed] [Google Scholar]
- Bessey P. Q., Watters J. M., Aoki T. T., Wilmore D. W. Combined hormonal infusion simulates the metabolic response to injury. Ann Surg. 1984 Sep;200(3):264–281. doi: 10.1097/00000658-198409000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas R. G., Humberstone D. A., Haystead A., Shaw J. H. Metabolic effects of recombinant human growth hormone: isotopic studies in the postabsorptive state and during total parenteral nutrition. Br J Surg. 1990 Jul;77(7):785–790. doi: 10.1002/bjs.1800770722. [DOI] [PubMed] [Google Scholar]
- Evans R. D., Argilés J. M., Williamson D. H. Metabolic effects of tumour necrosis factor-alpha (cachectin) and interleukin-1. Clin Sci (Lond) 1989 Oct;77(4):357–364. doi: 10.1042/cs0770357. [DOI] [PubMed] [Google Scholar]
- FAWCETT J. K., SCOTT J. E. A rapid and precise method for the determination of urea. J Clin Pathol. 1960 Mar;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Shires G. T., Lowry S. F. The biologic characteristics of cytokines and their implication in surgical injury. Surg Gynecol Obstet. 1990 Apr;170(4):363–378. [PubMed] [Google Scholar]
- Fuessl H. S., Burrin J. M., Williams G., Adrian T. E., Bloom S. R. The effect of a long-acting somatostatin analogue (SMS 201-995) on intermediary metabolism and gut hormones after a test meal in normal subjects. Aliment Pharmacol Ther. 1987 Aug;1(4):321–330. doi: 10.1111/j.1365-2036.1987.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Gelfand R. A., Matthews D. E., Bier D. M., Sherwin R. S. Role of counterregulatory hormones in the catabolic response to stress. J Clin Invest. 1984 Dec;74(6):2238–2248. doi: 10.1172/JCI111650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünfeld J. P., Eloy L., Araujo A., Russo-Marie F. Effects of gluco- and antiglucocorticoids on renal and aortic prostaglandin synthesis. Am J Physiol. 1986 Nov;251(5 Pt 2):F810–F816. doi: 10.1152/ajprenal.1986.251.5.F810. [DOI] [PubMed] [Google Scholar]
- Göschke H., Bär E., Girard J., Leutenegger A., Niederer W., Oberholzer M., Wolff G. Glucagon, insulin, cortisol, and growth hormone levels following major surgery: their relationship to glucose and free fatty acid elevations. Horm Metab Res. 1978 Nov;10(6):465–470. doi: 10.1055/s-0028-1093372. [DOI] [PubMed] [Google Scholar]
- Göschke H., Bär E., Girard J., Leutenegger A., Niederer W., Oberholzer M., Wolff G. Glucagon, insulin, cortisol, and growth hormone levels following major surgery: their relationship to glucose and free fatty acid elevations. Horm Metab Res. 1978 Nov;10(6):465–470. doi: 10.1055/s-0028-1093372. [DOI] [PubMed] [Google Scholar]
- Hansen B. A., Krog B., Vilstrup H. Insulin and glucose decreases the capacity of urea-N synthesis in the rat. Scand J Clin Lab Invest. 1986 Oct;46(6):599–603. doi: 10.3109/00365518609083719. [DOI] [PubMed] [Google Scholar]
- Hansen B. A., Vilstrup H. A method for determination of the capacity of urea synthesis in the rat. Scand J Clin Lab Invest. 1985 Jun;45(4):315–320. doi: 10.3109/00365518509161013. [DOI] [PubMed] [Google Scholar]
- Hausmann D. F., Nutz V., Rommelsheim K., Caspari R., Mosebach K. O. Anabolic steroids in polytrauma patients. Influence on renal nitrogen and amino acid losses: a double-blind study. JPEN J Parenter Enteral Nutr. 1990 Mar-Apr;14(2):111–114. doi: 10.1177/0148607190014002111. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Determination of total serum insulin (IRI) in insulin-treated diabetic patients. Diabetologia. 1972 Aug;8(4):260–266. doi: 10.1007/BF01225569. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of pancreatic and gut glucagon in plasma. Diabetologia. 1971 Feb;7(1):10–19. doi: 10.1007/BF02346248. [DOI] [PubMed] [Google Scholar]
- Heindorff H., Schulze S., Mogensen T., Almdal T., Kehlet H., Vilstrup H. Hormonal and neural blockade prevents the postoperative increase in amino acid clearance and urea synthesis. Surgery. 1992 May;111(5):543–550. [PubMed] [Google Scholar]
- Heindorff H., Vilstrup H., Almdal T., Harvald T., Nielsen J., Dalsgaard S. Elective cholecystectomy increases plasma amino-acid clearance and hepatic capacity for urea synthesis for one week. Clin Nutr. 1991 Feb;10(1):10–17. doi: 10.1016/0261-5614(91)90075-n. [DOI] [PubMed] [Google Scholar]
- Heindorff H., Vilstrup H., Bucher D., Billesbølle P., Thygesen V. Increased hepatic amino nitrogen conversion after elective cholecystectomy in man. Clin Sci (Lond) 1988 May;74(5):539–545. doi: 10.1042/cs0740539. [DOI] [PubMed] [Google Scholar]
- Kehlet H. Surgical stress: the role of pain and analgesia. Br J Anaesth. 1989 Aug;63(2):189–195. doi: 10.1093/bja/63.2.189. [DOI] [PubMed] [Google Scholar]
- Langhoff E., Flachs H., Ladefoged J., Hvidberg E. F. Intra-individual consistency of prednisolone kinetics during long-term prednisone treatment. Eur J Clin Pharmacol. 1984;26(5):651–653. doi: 10.1007/BF00543505. [DOI] [PubMed] [Google Scholar]
- Marco J., Calle C., Román D., Díaz-Fierros M., Villanueva M. L., Valverde I. Hyperglucagonism induced by glucocorticoid treatment in man. N Engl J Med. 1973 Jan 18;288(3):128–131. doi: 10.1056/NEJM197301182880305. [DOI] [PubMed] [Google Scholar]
- Mealy K., van Lanschot J. J., Robinson B. G., Rounds J., Wilmore D. W. Are the catabolic effects of tumor necrosis factor mediated by glucocorticoids? Arch Surg. 1990 Jan;125(1):42–48. doi: 10.1001/archsurg.1990.01410130044006. [DOI] [PubMed] [Google Scholar]
- Meguid M. M., Brennan M. F., Aoki T. T., Muller W. A., Ball M. R., Moore F. D. Hormone-substrate interrelationships following trauma. Arch Surg. 1974 Dec;109(6):776–783. doi: 10.1001/archsurg.1974.01360060046013. [DOI] [PubMed] [Google Scholar]
- Merkel C., Gatta A., Caregaro L., Sacerdoti D., Rondana M., Ruol A. Effect of somatostatin on liver blood flow and liver metabolic activity in patients with cirrhosis. Scand J Clin Lab Invest. 1987 Nov;47(7):667–672. [PubMed] [Google Scholar]
- Nordenström J., Sonnenfeld T., Arner P. Characterization of insulin resistance after surgery. Surgery. 1989 Jan;105(1):28–35. [PubMed] [Google Scholar]
- Radcliffe A., Johnson A., Dudley H. A. The effect of different calorific doses of carbohydrate on nitrogen excretion after surgery. Br J Surg. 1980 Jul;67(7):462–463. doi: 10.1002/bjs.1800670703. [DOI] [PubMed] [Google Scholar]
- Reeds P. J., Palmer R. M. Changes in prostaglandin release associated with inhibition of muscle protein synthesis by dexamethasone. Biochem J. 1984 May 1;219(3):953–957. doi: 10.1042/bj2190953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg H., Håkanson E., Anderberg B., Jorfeldt L., Schildt B., Tegler L. Thyroid hormones, catecholamine and cortisol concentrations after upper abdominal surgery. Acta Chir Scand. 1984;150(4):273–278. [PubMed] [Google Scholar]
- Sigsgaard I., Almdal T., Hansen B. A., Vilstrup H. Dexamethasone increases the capacity of urea synthesis time dependently and reduces the body weight of rats. Liver. 1988 Aug;8(4):193–197. doi: 10.1111/j.1600-0676.1988.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Vilstrup H. Effects of glucose on alanine-derived urea synthesis. Clin Physiol. 1984 Dec;4(6):495–507. doi: 10.1111/j.1475-097x.1984.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Vilstrup H., Hansen B. A., Almdal T. P. Glucagon increases hepatic efficacy for urea synthesis. J Hepatol. 1990 Jan;10(1):46–50. doi: 10.1016/0168-8278(90)90072-y. [DOI] [PubMed] [Google Scholar]
- Wahren J., Eriksson L. S. The influence of a long-acting somatostatin analogue on splanchnic haemodynamics and metabolism in healthy subjects and patients with liver cirrhosis. Scand J Gastroenterol Suppl. 1986;119:103–108. doi: 10.3109/00365528609087437. [DOI] [PubMed] [Google Scholar]
- Watters J. M., Bessey P. Q., Dinarello C. A., Wolff S. M., Wilmore D. W. Both inflammatory and endocrine mediators stimulate host responses to sepsis. Arch Surg. 1986 Feb;121(2):179–190. doi: 10.1001/archsurg.1986.01400020065008. [DOI] [PubMed] [Google Scholar]
- Wolfe B. M., Culebras J. M., Aoki T. T., O'Connor N. E., Finley R. J., Kaczowka A., Moore F. D. The effects of glucagon on protein metabolism in normal man. Surgery. 1979 Aug;86(2):248–257. [PubMed] [Google Scholar]


