Abstract
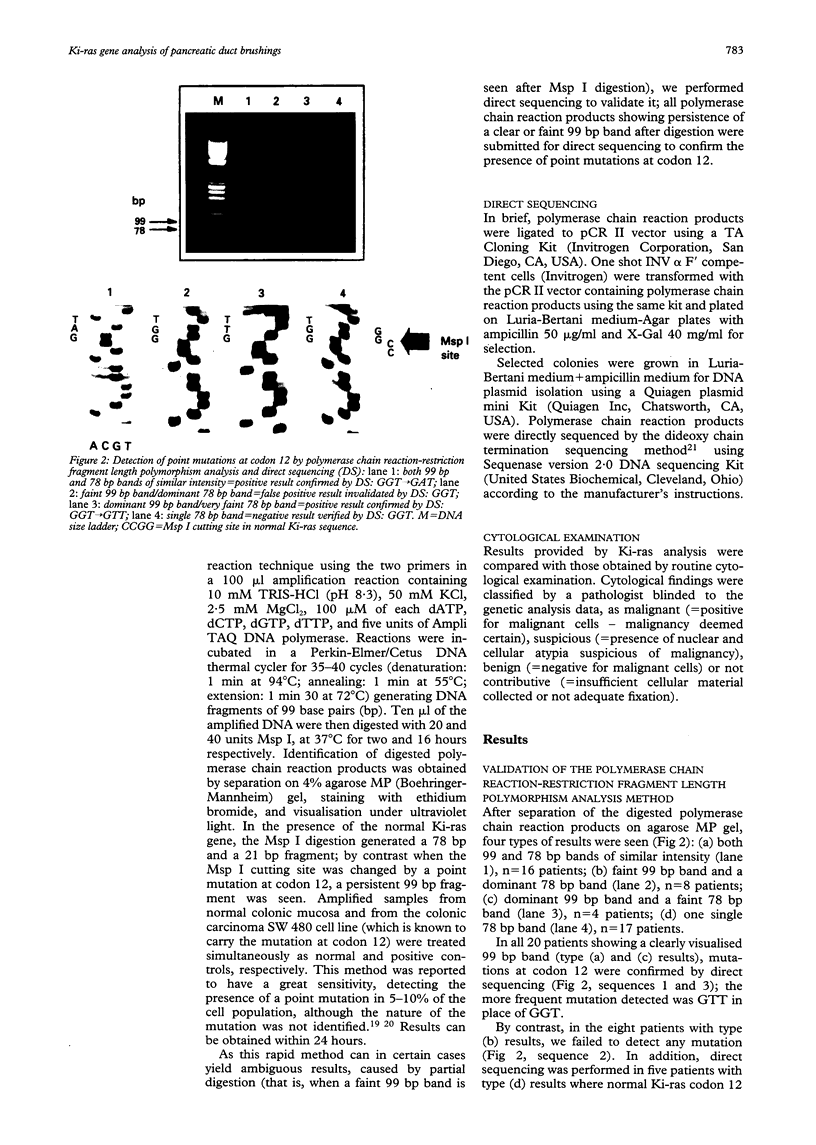
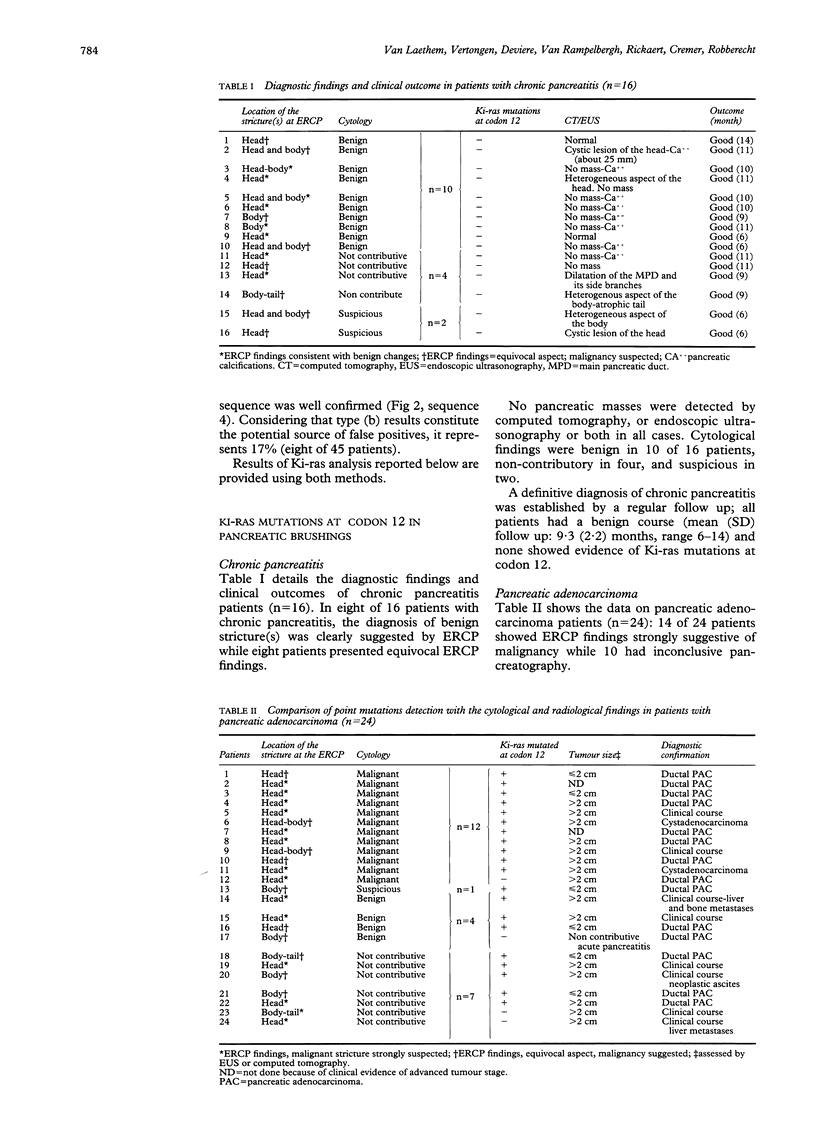
Differential diagnosis of pancreatic cancer and chronic pancreatitis is sometimes difficult and cytological examination of brushings or aspirated material collected during endoscopic retrograde cholangiopancreatography (ERCP) remains disappointing. As point mutations in codon 12 of the c-Ki-ras 2 gene are found in most pancreatic adenocarcinoma and not in chronic pancreatitis, this study analysed prospectively the presence of these mutations in brushing samples collected during ERCP in 45 patients (26 males, 19 females) showing a dominant stricture of the main pancreatic duct at pancreatography: 24 with pancreatic adenocarcinoma, 16 with chronic pancreatitis, and five intraductal mucin hypersecreting neoplasms. Twenty of 45 patients presented equivocal ERCP findings that did not permit a definite diagnosis. Ki-ras mutations at codon 12 were detected using a rapid and sensitive method based on polymerase chain reaction mediated restriction fragment length polymorphism analysis and confirmed by direct sequencing of polymerase chain reaction products. Results were compared with those provided by routine brush cytology. A definitive diagnosis was established for each patient. Mutations were detected in 20 of 24 patients with pancreatic adenocarcinoma (83%), but in none of the chronic pancreatitis patients and intraductal mucin hypersecreting neoplasms, irrespective of their location. By contrast, only 13 of 24 pancreatic adenocarcinoma (54%) were detected by conventional cytological examination, which yielded four false negative and seven non-contributive results. Sensitivity, specificity, and accuracy of molecular biological and cytological methods were 83%-76%, 100-83%, and 90%-58%, respectively. Notably the mutations could be detected in six patients with small tumour size (< or = 2 cm). In conclusion, Ki-ras analysis performed on pancreatic brushing samples is an efficient procedure, more accurate than cytology in the diagnosis of pancreatic adenocarcinoma, and highly specific in the differentiation between neoplastic and chronic inflammatory ductal changes, especially in patients showing inconclusive ERCP findings.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agostini S., Choux R., Payan M. J., Sastre B., Sahel J., Clement J. P. Mucinous pancreatic duct ectasia in the body of the pancreas. Radiology. 1989 Mar;170(3 Pt 1):815–816. doi: 10.1148/radiology.170.3.2916036. [DOI] [PubMed] [Google Scholar]
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Ando M., Takemura K., Maruyama M., Endo M., Iwama T., Yuasa Y. Mutations in c-K-ras 2 gene codon 12 during colorectal tumorigenesis in familial adenomatous polyposis. Gastroenterology. 1992 Dec;103(6):1725–1731. doi: 10.1016/0016-5085(92)91427-6. [DOI] [PubMed] [Google Scholar]
- Cerny W. L., Mangold K. A., Scarpelli D. G. K-ras mutation is an early event in pancreatic duct carcinogenesis in the Syrian golden hamster. Cancer Res. 1992 Aug 15;52(16):4507–4513. [PubMed] [Google Scholar]
- Goodale R. L., Gajl-Peczalska K., Dressel T., Samuelson J. Cytologic studies for the diagnosis of pancreatic cancer. Cancer. 1981 Mar 15;47(6 Suppl):1652–1655. doi: 10.1002/1097-0142(19810315)47:6+<1652::aid-cncr2820471432>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Gudjonsson B. Cancer of the pancreas. 50 years of surgery. Cancer. 1987 Nov 1;60(9):2284–2303. doi: 10.1002/1097-0142(19871101)60:9<2284::aid-cncr2820600930>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Haliassos A., Chomel J. C., Grandjouan S., Kruh J., Kaplan J. C., Kitzis A. Detection of minority point mutations by modified PCR technique: a new approach for a sensitive diagnosis of tumor-progression markers. Nucleic Acids Res. 1989 Oct 25;17(20):8093–8099. doi: 10.1093/nar/17.20.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine N. R., Jain S., Hughes C. M., Staddon S. L., Maillet B., Hall P. A., Klöppel G. Ki-ras oncogene activation in preinvasive pancreatic cancer. Gastroenterology. 1992 Jan;102(1):230–236. doi: 10.1016/0016-5085(92)91805-e. [DOI] [PubMed] [Google Scholar]
- Lowenfels A. B., Maisonneuve P., Cavallini G., Ammann R. W., Lankisch P. G., Andersen J. R., Dimagno E. P., Andrén-Sandberg A., Domellöf L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993 May 20;328(20):1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Smith D. H., Chen E. Y., Seeburg P. H., Goeddel D. V., Levinson A. D. Structure and organization of the human Ki-ras proto-oncogene and a related processed pseudogene. Nature. 1983 Aug 11;304(5926):501–506. doi: 10.1038/304501a0. [DOI] [PubMed] [Google Scholar]
- Morohoshi T., Kanda M., Asanuma K., Klöppel G. Intraductal papillary neoplasms of the pancreas. A clinicopathologic study of six patients. Cancer. 1989 Sep 15;64(6):1329–1335. doi: 10.1002/1097-0142(19890915)64:6<1329::aid-cncr2820640627>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Nakaizumi A., Tatsuta M., Uehara H., Yamamoto R., Takenaka A., Kishigami Y., Takemura K., Kitamura T., Okuda S. Cytologic examination of pure pancreatic juice in the diagnosis of pancreatic carcinoma. The endoscopic retrograde intraductal catheter aspiration cytologic technique. Cancer. 1992 Dec 1;70(11):2610–2614. doi: 10.1002/1097-0142(19921201)70:11<2610::aid-cncr2820701107>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Niederau C., Grendell J. H. Diagnosis of pancreatic carcinoma. Imaging techniques and tumor markers. Pancreas. 1992;7(1):66–86. doi: 10.1097/00006676-199201000-00011. [DOI] [PubMed] [Google Scholar]
- Palazzo L., Roseau G., Gayet B., Vilgrain V., Belghiti J., Fékéte F., Paolaggi J. A. Endoscopic ultrasonography in the diagnosis and staging of pancreatic adenocarcinoma. Results of a prospective study with comparison to ultrasonography and CT scan. Endoscopy. 1993 Feb;25(2):143–150. doi: 10.1055/s-2007-1010273. [DOI] [PubMed] [Google Scholar]
- Rösch T., Braig C., Gain T., Feuerbach S., Siewert J. R., Schusdziarra V., Classen M. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology. 1992 Jan;102(1):188–199. doi: 10.1016/0016-5085(92)91800-j. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh E., Czerniak A., Nass S., Klein E. Role of endoscopic retrograde cholangiopancreatography in differentiating pancreatic cancer coexisting with chronic pancreatitis. Cancer. 1990 Feb 15;65(4):893–896. doi: 10.1002/1097-0142(19900215)65:4<893::aid-cncr2820650412>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Shibata D., Almoguera C., Forrester K., Dunitz J., Martin S. E., Cosgrove M. M., Perucho M., Arnheim N. Detection of c-K-ras mutations in fine needle aspirates from human pancreatic adenocarcinomas. Cancer Res. 1990 Feb 15;50(4):1279–1283. [PubMed] [Google Scholar]
- Tada M., Omata M., Kawai S., Saisho H., Ohto M., Saiki R. K., Sninsky J. J. Detection of ras gene mutations in pancreatic juice and peripheral blood of patients with pancreatic adenocarcinoma. Cancer Res. 1993 Jun 1;53(11):2472–2474. [PubMed] [Google Scholar]
- Tada M., Omata M., Ohto M. Clinical application of ras gene mutation for diagnosis of pancreatic adenocarcinoma. Gastroenterology. 1991 Jan;100(1):233–238. doi: 10.1016/0016-5085(91)90606-l. [DOI] [PubMed] [Google Scholar]
- Tada M., Omata M., Ohto M. Ras gene mutations in intraductal papillary neoplasms of the pancreas. Analysis in five cases. Cancer. 1991 Feb 1;67(3):634–637. doi: 10.1002/1097-0142(19910201)67:3<634::aid-cncr2820670318>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Venu R. P., Geenen J. E., Kini M., Hogan W. J., Payne M., Johnson G. K., Schmalz M. J. Endoscopic retrograde brush cytology. A new technique. Gastroenterology. 1990 Nov;99(5):1475–1479. doi: 10.1016/0016-5085(90)91178-9. [DOI] [PubMed] [Google Scholar]
- Yanagisawa A., Kato Y., Ohtake K., Kitagawa T., Ohashi K., Hori M., Takagi K., Sugano H. c-Ki-ras point mutations in ductectatic-type mucinous cystic neoplasms of the pancreas. Jpn J Cancer Res. 1991 Oct;82(10):1057–1060. doi: 10.1111/j.1349-7006.1991.tb01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa A., Ohtake K., Ohashi K., Hori M., Kitagawa T., Sugano H., Kato Y. Frequent c-Ki-ras oncogene activation in mucous cell hyperplasias of pancreas suffering from chronic inflammation. Cancer Res. 1993 Mar 1;53(5):953–956. [PubMed] [Google Scholar]