Abstract
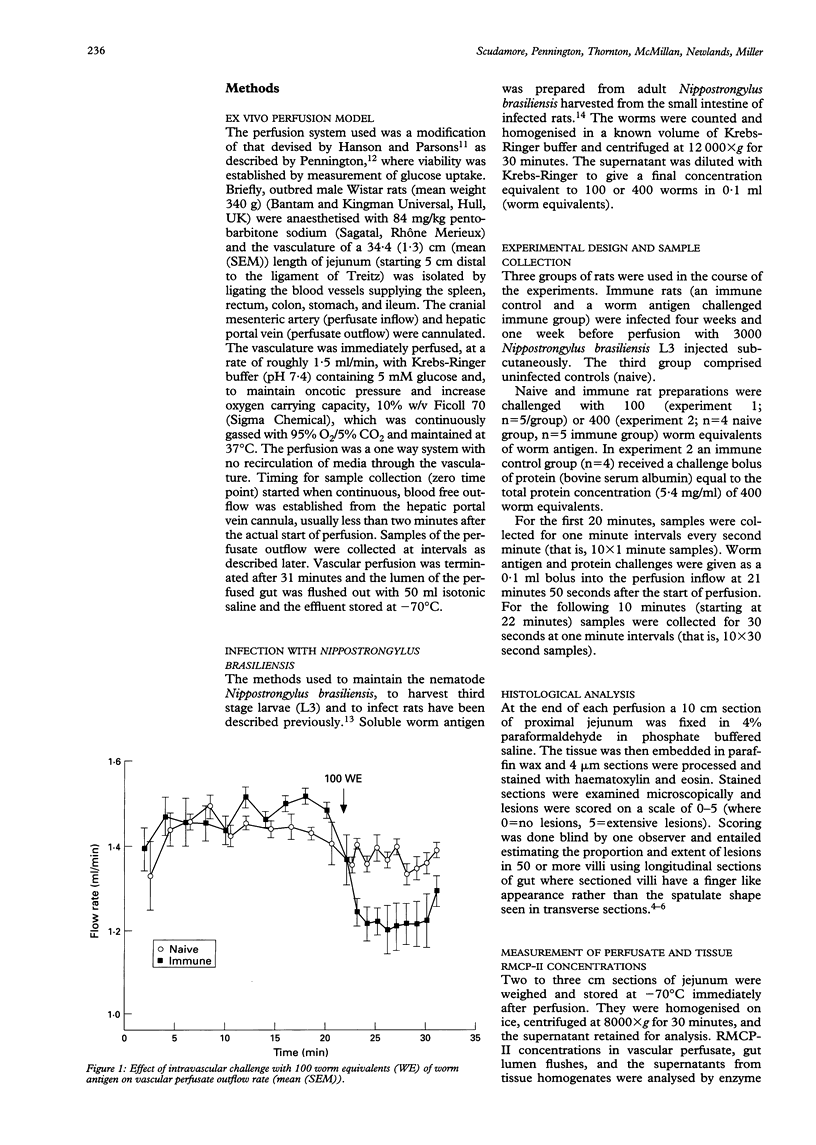
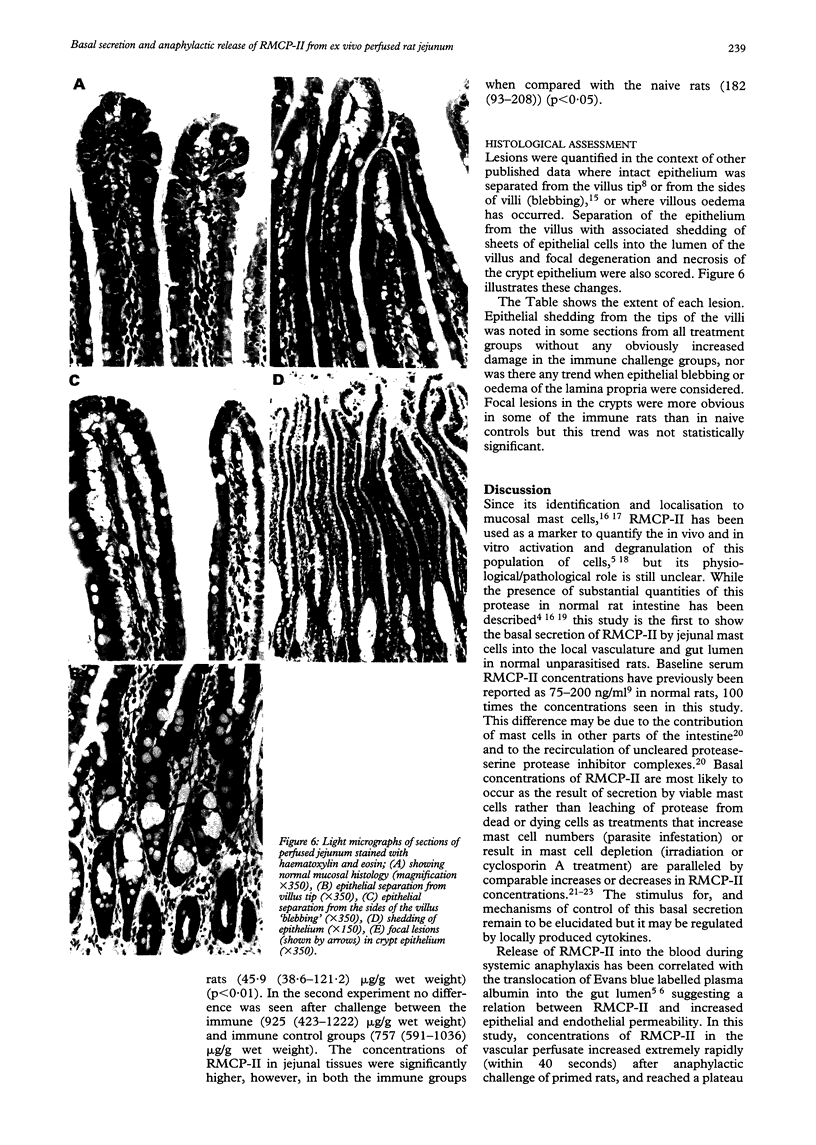
The kinetics of the release of rat mast cell protease-II (RMCP-II) from mucosal mast cells in the jejunum of Nippostrongylus brasiliensis primed (immune) rats was investigated using ex vivo perfusion of a segment of jejunum through the cranial mesenteric artery. The aim of the study was to assess the role of the protease in anaphylaxis and in particular to ascertain whether it is responsible for the histological changes, which include widespread epithelial shedding, seen in the mucosa in in vivo models of anaphylaxis. Perfusion of the jejunal vasculature with a Krebs-Ringer buffer showed that there was basal secretion of RMCP-II by jejunal mast cells in all rats studied. The baseline concentration of RMCP-II was significantly greater (p < 0.05) in immune rats (> 7 ng/ml) previously exposed to nippostrongylus infection than in control, naive animals (< 2 ng/ml). Challenge of immune rats with 100 or 400 worm equivalents of whole worm antigen resulted in an immediate (within 40 seconds) and significant (p < 0.02) increase in the concentration of RMCP-II (to > 3 micrograms/ml) in the vascular perfusate, which was not seen in naive rats or immune rats challenged with an irrelevant antigen. Greater amounts of RMCP-II were also recovered from the jejunal lumen of immune rats compared with naive rats after challenge of both groups with worm antigen. Despite the release of microgram quantities of RMCP-II into the gut lumen and vascular perfusate, however, there were no significant changes seen in the mucosal histology. These results suggest that RMCP-II alone is not responsible fore the loss of gut epithelium seen during anaphylaxis in the rat.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crowe S. E., Perdue M. H. Functional abnormalities in the intestine associated with mucosal mast cell activation. Reg Immunol. 1992 Mar-Apr;4(2):113–117. [PubMed] [Google Scholar]
- Crowe S. E., Perdue M. H. Gastrointestinal food hypersensitivity: basic mechanisms of pathophysiology. Gastroenterology. 1992 Sep;103(3):1075–1095. doi: 10.1016/0016-5085(92)90047-3. [DOI] [PubMed] [Google Scholar]
- Cummins A. G., Munro G. H., Huntley J. F., Miller H. R., Ferguson A. Separate effects of irradiation and of graft-versus-host reaction on rat mucosal mast cells. Gut. 1989 Mar;30(3):355–360. doi: 10.1136/gut.30.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins A. G., Munro G. H., Miller H. R., Ferguson A. Effect of cyclosporin A treatment on the enteropathy of graft-versus-host reaction in the rat: a quantitative study of intestinal morphology, epithelial cell kinetics and mucosal immune activity. Immunol Cell Biol. 1989 Jun;67(Pt 3):153–160. doi: 10.1038/icb.1989.25. [DOI] [PubMed] [Google Scholar]
- Fernández-Gallardo S., Gijón M. A., García C., Furio V., Liu F. T., Sánchez Crespo M. The role of platelet-activating factor and peptidoleukotrienes in the vascular changes of rat passive anaphylaxis. Br J Pharmacol. 1992 Jan;105(1):119–125. doi: 10.1111/j.1476-5381.1992.tb14221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P. J., Parsons D. S. The utilization of glucose and production of lactate by in vitro preparations of rat small intestine: effects of vascular perfusion. J Physiol. 1976 Mar;255(3):775–795. doi: 10.1113/jphysiol.1976.sp011307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley J. F., Mackellar A., Newlands G. F., Irvine J., Miller H. R. Mapping of the rat mast cell granule proteinases RMCPI and II by enzyme-linked immunosorbent assay and paired immunofluorescence. APMIS. 1990 Oct;98(10):933–944. doi: 10.1111/j.1699-0463.1990.tb05018.x. [DOI] [PubMed] [Google Scholar]
- King S. J., Miller H. R. Anaphylactic release of mucosal mast cell protease and its relationship to gut permeability in Nippostrongylus-primed rats. Immunology. 1984 Apr;51(4):653–660. [PMC free article] [PubMed] [Google Scholar]
- King S. J., Miller H. R., Woodbury R. G., Newlands G. F. Gut mucosal mast cells in Nippostrongylus-primed rats are the major source of secreted rat mast cell protease II following systemic anaphylaxis. Eur J Immunol. 1986 Feb;16(2):151–155. doi: 10.1002/eji.1830160208. [DOI] [PubMed] [Google Scholar]
- Leng W., Kuo C. G., Qureshi R., Jakschik B. A. Role of leukotrienes in vascular changes in the rat mesentery and skin in anaphylaxis. J Immunol. 1988 Apr 1;140(7):2361–2368. [PubMed] [Google Scholar]
- MacDonald A. J., Haig D. M., Bazin H., McGuigan A. C., Moqbel R., Miller H. R. IgE-mediated release of rat mast cell protease II, beta-hexosaminidase and leukotriene C4 from cultured bone marrow-derived rat mast cells. Immunology. 1989 Jul;67(3):414–418. [PMC free article] [PubMed] [Google Scholar]
- MacNaughton W. K., Leach K. E., Prud'homme-Lalonde L., Ho W., Sharkey K. A. Ionizing radiation reduces neurally evoked electrolyte transport in rat ileum through a mast cell-dependent mechanism. Gastroenterology. 1994 Feb;106(2):324–335. doi: 10.1016/0016-5085(94)90589-4. [DOI] [PubMed] [Google Scholar]
- Miller H. R., Huntley J. F., Newlands G. F., Irvine J. Granule chymases and the characterization of mast cell phenotype and function in rat and mouse. Monogr Allergy. 1990;27:1–30. [PubMed] [Google Scholar]
- Miller H. R., Woodbury R. G., Huntley J. F., Newlands G. Systemic release of mucosal mast-cell protease in primed rats challenged with Nippostrongylus brasiliensis. Immunology. 1983 Jul;49(3):471–479. [PMC free article] [PubMed] [Google Scholar]
- Nawa Y., Miller H. R., Hall E., Jarrett E. E. Adoptive transfer of total and parasite-specific IgE responses in rats infected with Nippostrongylus brasiliensis. Immunology. 1981 Sep;44(1):119–123. [PMC free article] [PubMed] [Google Scholar]
- Nawa Y., Miller H. R. Protection against Nippostrongylus brasiliensis by adoptive immunization with immune thoracic duct lymphocytes. Cell Immunol. 1978 Apr;37(1):51–60. doi: 10.1016/0008-8749(78)90173-9. [DOI] [PubMed] [Google Scholar]
- Patrick M. K., Dunn I. J., Buret A., Miller H. R., Huntley J. F., Gibson S., Gall D. G. Mast cell protease release and mucosal ultrastructure during intestinal anaphylaxis in the rat. Gastroenterology. 1988 Jan;94(1):1–9. doi: 10.1016/0016-5085(88)90603-8. [DOI] [PubMed] [Google Scholar]
- Pennington A. M., Corpe C. P., Kellett G. L. Rapid regulation of rat jejunal glucose transport by insulin in a luminally and vascularly perfused preparation. J Physiol. 1994 Jul 15;478(Pt 2):187–193. doi: 10.1113/jphysiol.1994.sp020241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson C. G., Gustafsson B., Erjefält J. S., Sundler F. Mucosal exudation of plasma is a noninjurious intestinal defense mechanism. Allergy. 1993 Nov;48(8):581–586. doi: 10.1111/j.1398-9995.1993.tb00752.x. [DOI] [PubMed] [Google Scholar]
- Razin E., Mencia-Huerta J. M., Stevens R. L., Lewis R. A., Liu F. T., Corey E., Austen K. F. IgE-mediated release of leukotriene C4, chondroitin sulfate E proteoglycan, beta-hexosaminidase, and histamine from cultured bone marrow-derived mouse mast cells. J Exp Med. 1983 Jan 1;157(1):189–201. doi: 10.1084/jem.157.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe B., Haraldsson B. Transport of macromolecules across microvascular walls: the two-pore theory. Physiol Rev. 1994 Jan;74(1):163–219. doi: 10.1152/physrev.1994.74.1.163. [DOI] [PubMed] [Google Scholar]
- Saarinen J., Kalkkinen N., Welgus H. G., Kovanen P. T. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J Biol Chem. 1994 Jul 8;269(27):18134–18140. [PubMed] [Google Scholar]
- Sage H., Woodbury R. G., Bornstein P. Structural studies on human type IV collagen. J Biol Chem. 1979 Oct 10;254(19):9893–9900. [PubMed] [Google Scholar]
- Sanderson I. R., Walker W. A. Uptake and transport of macromolecules by the intestine: possible role in clinical disorders (an update). Gastroenterology. 1993 Feb;104(2):622–639. doi: 10.1016/0016-5085(93)90436-g. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Gruzenski G. M., Lagunoff D. Immunofluorescent localization of a serine protease in rat small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2785–2789. doi: 10.1073/pnas.75.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury R. G., Miller H. R., Huntley J. F., Newlands G. F., Palliser A. C., Wakelin D. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. 1984 Nov 29-Dec 5Nature. 312(5993):450–452. doi: 10.1038/312450a0. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Neurath H. Purification of an atypical mast cell protease and its levels in developing rats. Biochemistry. 1978 Oct 3;17(20):4298–4304. doi: 10.1021/bi00613a029. [DOI] [PubMed] [Google Scholar]



