Abstract
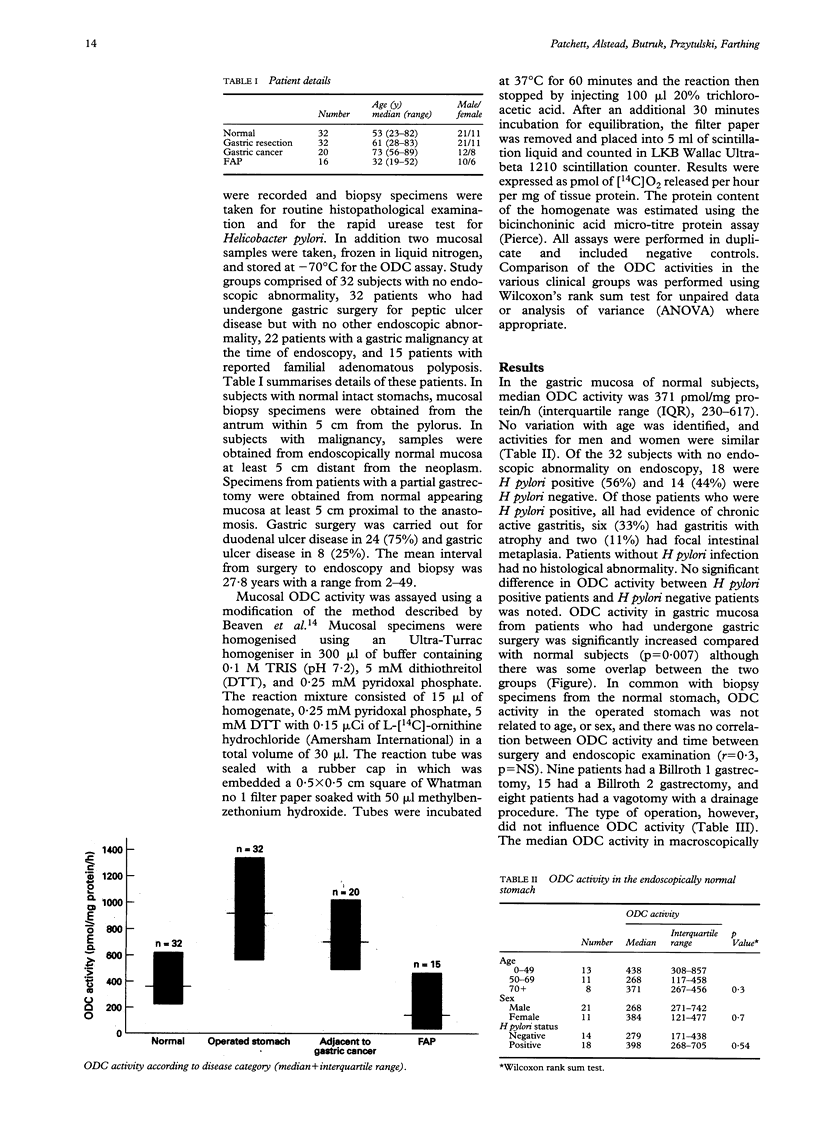
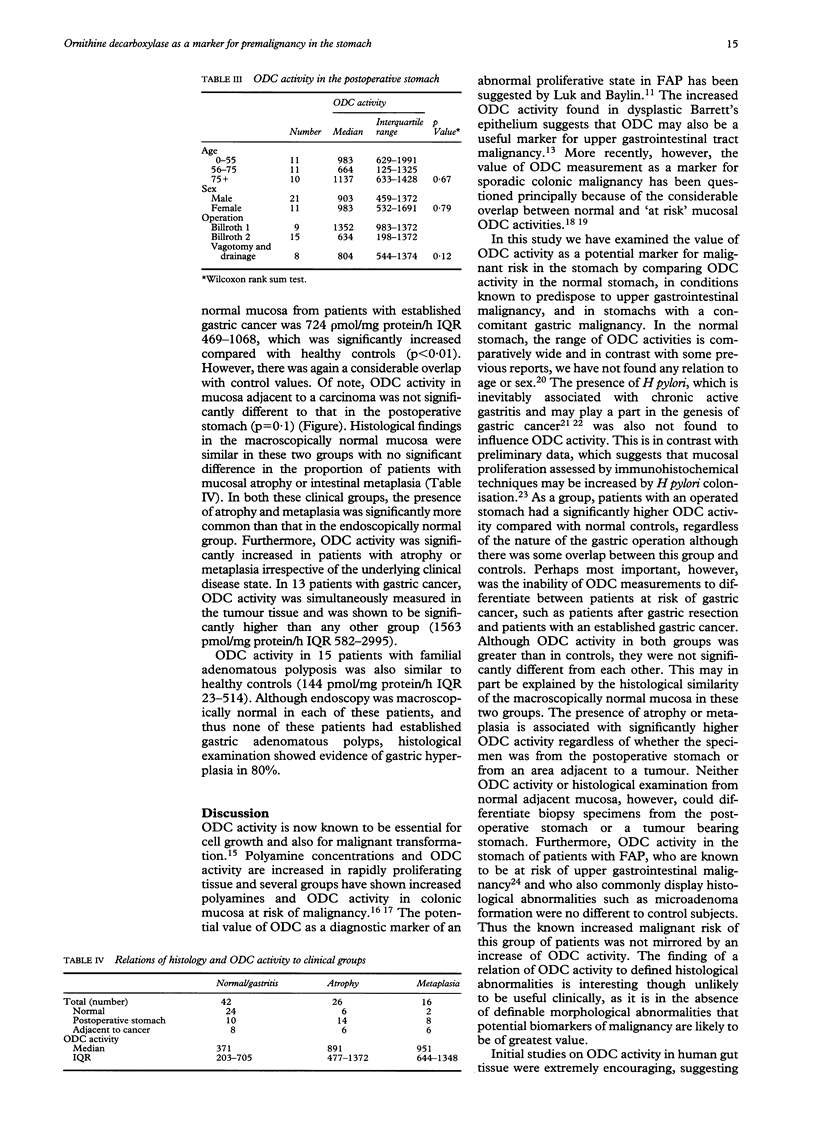
Assessment of mucosal ornithine decarboxylase (ODC) activity in the human large bowel may be of value as a marker of potential malignant risk. Its value as a marker of premalignancy in the upper gastrointestinal tract is less clear. Using a [14C]-ornithine bioassay, gastric mucosal ODC activity was measured in 32 normal subjects and 22 patients with confirmed gastric cancer. These results were compared with 47 patients at increased risk of upper gastrointestinal malignancy, (32 patients with partial gastric resection, 15 patients with familial adenomatous polyposis). Median ODC activity in normal subjects was 371 pmol/mg protein/h, (interquartile range (IQR), 230-617). There was no variation with age or sex and no relation to Helicobacter pylori status. Normal subjects had significantly lower ODC activity than patients with a gastric resection or confirmed gastric cancer, but similar to patients with familial adenomatous polyposis. Furthermore, no difference in activity was identified between patients with a gastric resection and established gastric cancer. ODC activity was, however, significantly increased in areas of gastric atrophy or intestinal metaplasia, regardless of the clinical group from which the samples were obtained. It is concluded that measurement of mucosal ODC activity does not provide additional predictive information of malignant risk in the stomach and investigation of other potential biomarkers of malignancy is warranted.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auvinen M., Paasinen A., Andersson L. C., Hölttä E. Ornithine decarboxylase activity is critical for cell transformation. Nature. 1992 Nov 26;360(6402):355–358. doi: 10.1038/360355a0. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Wilcox G., Terpstra G. K. A microprocedure for the measurement of 14CO2 release from [14C]carboxyl-labeled amino acids. Anal Biochem. 1978 Feb;84(2):638–641. doi: 10.1016/0003-2697(78)90089-1. [DOI] [PubMed] [Google Scholar]
- Bodmer W. F., Bailey C. J., Bodmer J., Bussey H. J., Ellis A., Gorman P., Lucibello F. C., Murday V. A., Rider S. H., Scambler P. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987 Aug 13;328(6131):614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- Cannon-Albright L. A., Skolnick M. H., Bishop D. T., Lee R. G., Burt R. W. Common inheritance of susceptibility to colonic adenomatous polyps and associated colorectal cancers. N Engl J Med. 1988 Sep 1;319(9):533–537. doi: 10.1056/NEJM198809013190902. [DOI] [PubMed] [Google Scholar]
- Colarian J., Arlow F. L., Calzada R., Luk G. D., Majumdar A. P. Differential activation of ornithine decarboxylase and tyrosine kinase in the rectal mucosa of patients with hyperplastic and adenomatous polyps. Gastroenterology. 1991 Jun;100(6):1528–1532. doi: 10.1016/0016-5085(91)90649-6. [DOI] [PubMed] [Google Scholar]
- Desai T. K., Parikh N., Bronstein J. C., Luk G. D., Bull A. W. Failure of rectal ornithine decarboxylase to identify adenomatous polyp status. Gastroenterology. 1992 Nov;103(5):1562–1567. doi: 10.1016/0016-5085(92)91178-7. [DOI] [PubMed] [Google Scholar]
- Fireman Z., Rozen P., Fine N., Chetrit A. Influence of demographic parameters on rectal epithelial proliferation. Cancer Lett. 1989 Sep 15;47(1-2):133–140. doi: 10.1016/0304-3835(89)90189-4. [DOI] [PubMed] [Google Scholar]
- Garewal H. S., Sampliner R., Alberts D., Steinbronn K. Increase in ornithine decarboxylase activity associated with development of dysplasia in Barrett's esophagus. Dig Dis Sci. 1989 Feb;34(2):312–314. doi: 10.1007/BF01536068. [DOI] [PubMed] [Google Scholar]
- Jagelman D. G., DeCosse J. J., Bussey H. J. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet. 1988 May 21;1(8595):1149–1151. doi: 10.1016/s0140-6736(88)91962-9. [DOI] [PubMed] [Google Scholar]
- Lipkin M. Biomarkers of increased susceptibility to gastrointestinal cancer. Their development and application to studies of cancer prevention. Gastroenterology. 1987 Apr;92(4):1083–1086. doi: 10.1016/0016-5085(87)90987-5. [DOI] [PubMed] [Google Scholar]
- Luk G. D., Baylin S. B. Ornithine decarboxylase as a biologic marker in familial colonic polyposis. N Engl J Med. 1984 Jul 12;311(2):80–83. doi: 10.1056/NEJM198407123110202. [DOI] [PubMed] [Google Scholar]
- Luk G. D., Silverman A. L., Giardiello F. M. Biochemical markers in patients with familial colonic neoplasia. Semin Surg Oncol. 1987;3(2):126–132. doi: 10.1002/ssu.2980030215. [DOI] [PubMed] [Google Scholar]
- Narisawa T., Takahashi M., Niwa M., Koyama H., Kotanagi H., Kusaka N., Yamazaki Y., Nagasawa O., Koyama K., Wakizaka A. Increased mucosal ornithine decarboxylase activity in large bowel with multiple tumors, adenocarcinoma, and adenoma. Cancer. 1989 Apr 15;63(8):1572–1576. doi: 10.1002/1097-0142(19890415)63:8<1572::aid-cncr2820630821>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Nomura A., Stemmermann G. N., Chyou P. H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991 Oct 17;325(16):1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Sasaki M., Sugio K., Sato C., Iwama T., Ikeuchi T., Tonomura A., Sasazuki T., Miyaki M. Loss of constitutional heterozygosity in colon carcinoma from patients with familial polyposis coli. Nature. 1988 Jan 21;331(6153):273–277. doi: 10.1038/331273a0. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Porter C. W., Herrera-Ornelas L., Pera P., Petrelli N. F., Mittelman A. Polyamine biosynthetic activity in normal and neoplastic human colorectal tissues. Cancer. 1987 Sep 15;60(6):1275–1281. doi: 10.1002/1097-0142(19870915)60:6<1275::aid-cncr2820600619>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Quirke P., Dixon M. F., Day D. W., Fozard J. B., Talbot I. C., Bird C. C. DNA aneuploidy and cell proliferation in familial adenomatous polyposis. Gut. 1988 May;29(5):603–607. doi: 10.1136/gut.29.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler R. S., Ulshen M. H., Lyles C. M., McAuliffe C. A., Fuller C. R. Rectal mucosal ornithine decarboxylase activity is not a useful marker of risk for colorectal neoplasia. Dig Dis Sci. 1992 Nov;37(11):1718–1724. doi: 10.1007/BF01299865. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- de Dombal F. T., Price A. B., Thompson H., Williams G. T., Morgan A. G., Softley A., Clamp S. E., Unwin B. J. The British Society of Gastroenterology early gastric cancer/dysplasia survey: an interim report. Gut. 1990 Jan;31(1):115–120. doi: 10.1136/gut.31.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]


