Abstract
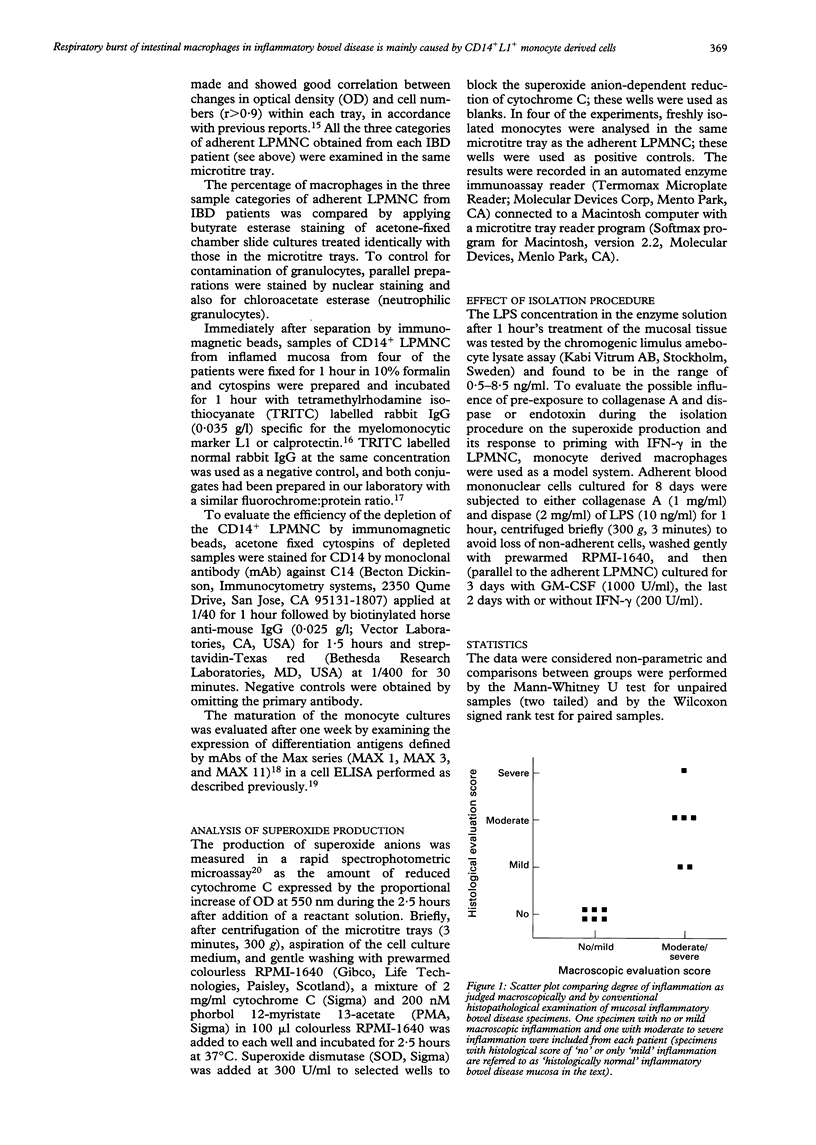
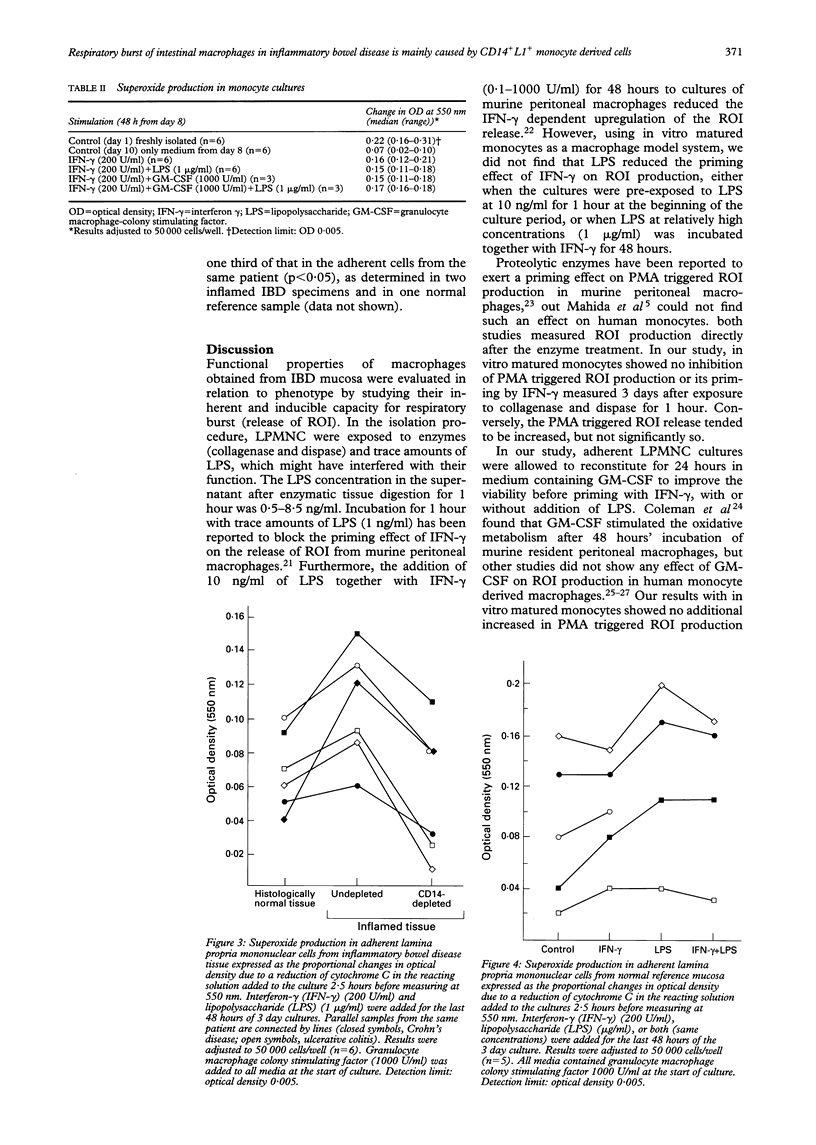
Macrophages play a crucial role in intestinal mucosal defence, forming dense subepithelial aggregates, particularly in the colon. One of their important bactericidal mechanisms is production of oxygen radicals but this may damage the intestinal epithelium, perhaps as an early step in inflammatory bowel disease (IBD). The potential for release of oxygen radicals from mucosal macrophages in IBD was measured and whether a difference exists between newly arrived (CD14+L1+) monocyte-like cells and resident macrophages (CD14(-)L1-), without or with additional priming in vitro, was investigated. Lamina propria mononuclear cells from six patients with IBD and five with a normal intestine were isolated with an ethylenediaminetetra acetic acid/collagenase/dispase technique and cultured for three days. The cells were tested with or without interferon gamma (200 U/ml) priming in the presence or absence of lipopolysaccharide (1 microgram/ml) for the last 48 hours in cultures. Samples from inflamed IBD mucosa depleted of CD14+ cells by immunomagnetic beads were compared with their undepleted counterparts and with samples from virtually normal mucosa from the same patients. The production of oxygen radicals was measured as the amount of reduced cytochrome C 2.5 hours after triggering with phorbol 12-myristate 13-acetate. The oxygen radical production in macrophages from moderately or severely inflamed mucosa was reduced by median 69% (range 22%-79%, p < 0.027) after depletion of CD14+ cells, reaching a level similar to that found for virtually normal samples from the same IBD patients. Furthermore, this production did not increase significantly in mucosal macrophages from normal reference mucosa and from virtually normal or inflamed IBD mucosa after priming with interferon gamma with or without addition of lipopolysaccharide. Upregulation of a respiratory burst in subepithelial resident macrophages os not a likely pathogenetic step in IBD. The increased oxygen radical production shown by macrophages from IBD lesions can, however, be ascribed to recently extravasated CD14+L1+ monocyte-like cells. Inhibition of extravasation of these reactive cells may form part of a therapeutic approach in the future.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. C., Cornwall S., Poulter L. W., Dhillon A. P., Pounder R. E. Macrophage heterogeneity in normal colonic mucosa and in inflammatory bowel disease. Gut. 1988 Nov;29(11):1531–1538. doi: 10.1136/gut.29.11.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreesen R., Mackensen A., Osterholz J., Brugger W., Löhr G. W. Microculture assay for human macrophage maturation in vitro. Cell-ELISA analysis of differentiation antigen expression. Int Arch Allergy Appl Immunol. 1988;86(3):281–287. doi: 10.1159/000234585. [DOI] [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Amrani D., Mosesson M. W., Bianco C. Receptors for cold-insoluble globulin (plasma fibronectin) on human monocytes. J Exp Med. 1981 Jan 1;153(1):42–60. doi: 10.1084/jem.153.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Conjugates of immunoglobulin G with different fluorochromes. I. Characterization by anionic-exchange chromatography. Scand J Immunol. 1973;2(3):273–290. doi: 10.1111/j.1365-3083.1973.tb02037.x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Dale I., Fagerhol M. K. Distribution of a formalin-resistant myelomonocytic antigen (L1) in human tissues. I. Comparison with other leukocyte markers by paired immunofluorescence and immunoenzyme staining. Am J Clin Pathol. 1987 Jun;87(6):681–699. doi: 10.1093/ajcp/87.6.681. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components. Immunohistochemistry with a cold ethanol-fixation technique. Immunology. 1974 Jun;26(6):1101–1114. [PMC free article] [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. L., Chodakewitz J. A., Bartiss A. H., Mellors J. W. Granulocyte-macrophage colony-stimulating factor enhances selective effector functions of tissue-derived macrophages. Blood. 1988 Aug;72(2):573–578. [PubMed] [Google Scholar]
- Dale I., Brandtzaeg P., Fagerhol M. K., Scott H. Distribution of a new myelomonocytic antigen (L1) in human peripheral blood leukocytes. Immunofluorescence and immunoperoxidase staining features in comparison with lysozyme and lactoferrin. Am J Clin Pathol. 1985 Jul;84(1):24–34. doi: 10.1093/ajcp/84.1.24. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F. Trace levels of bacterial lipopolysaccharide prevent interferon-gamma or tumor necrosis factor-alpha from enhancing mouse peritoneal macrophage respiratory burst capacity. J Immunol. 1987 Sep 15;139(6):1971–1977. [PubMed] [Google Scholar]
- Gillies R. J., Didier N., Denton M. Determination of cell number in monolayer cultures. Anal Biochem. 1986 Nov 15;159(1):109–113. doi: 10.1016/0003-2697(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Hogg N., MacDonald S., Slusarenko M., Beverley P. C. Monoclonal antibodies specific for human monocytes, granulocytes and endothelium. Immunology. 1984 Dec;53(4):753–767. [PMC free article] [PubMed] [Google Scholar]
- Hume D. A., Allan W., Hogan P. G., Doe W. F. Immunohistochemical characterisation of macrophages in human liver and gastrointestinal tract: expression of CD4, HLA-DR, OKM1, and the mature macrophage marker 25F9 in normal and diseased tissue. J Leukoc Biol. 1987 Nov;42(5):474–484. doi: 10.1002/jlb.42.5.474. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Chadwick D. A., Cohn Z. A. Priming of macrophages for enhanced oxidative metabolism by exposure to proteolytic enzymes. J Exp Med. 1981 Jun 1;153(6):1678–1683. doi: 10.1084/jem.153.6.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazmi A., Nielsen H., Hovgaard D., Borregaard N., Nissen N. I. Modulation of neutrophil and monocyte function by recombinant human granulocyte macrophage colony-stimulating factor in patients with lymphoma. Eur J Clin Invest. 1991 Apr;21(2):219–224. doi: 10.1111/j.1365-2362.1991.tb01813.x. [DOI] [PubMed] [Google Scholar]
- Kvale D., Brandtzaeg P., Løvhaug D. Up-regulation of the expression of secretory component and HLA molecules in a human colonic cell line by tumour necrosis factor-alpha and gamma interferon. Scand J Immunol. 1988 Sep;28(3):351–357. doi: 10.1111/j.1365-3083.1988.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Lepay D. A., Nathan C. F., Steinman R. M., Murray H. W., Cohn Z. A. Murine Kupffer cells. Mononuclear phagocytes deficient in the generation of reactive oxygen intermediates. J Exp Med. 1985 May 1;161(5):1079–1096. doi: 10.1084/jem.161.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. S., Gordon S. Secretion of plasminogen activator by bone marrow-derived mononuclear phagocytes and its enhancement by colony-stimulating factor. J Exp Med. 1979 Aug 1;150(2):231–245. doi: 10.1084/jem.150.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M., Martinache C., Canepa S., Chokri M., Scotto F., Bartholeyns J. Autologous lymphocytes prevent the death of monocytes in culture and promote, as do GM-CSF, IL-3 and M-CSF, their differentiation into macrophages. J Immunol Methods. 1993 Feb 26;159(1-2):29–38. doi: 10.1016/0022-1759(93)90138-w. [DOI] [PubMed] [Google Scholar]
- Mahida Y. R., Wu K. C., Jewell D. P. Characterization of antigen-presenting activity of intestinal mononuclear cells isolated from normal and inflammatory bowel disease colon and ileum. Immunology. 1988 Dec;65(4):543–549. [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Wu K. C., Jewell D. P. Respiratory burst activity of intestinal macrophages in normal and inflammatory bowel disease. Gut. 1989 Oct;30(10):1362–1370. doi: 10.1136/gut.30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Prendergast T. J., Wiebe M. E., Stanley E. R., Platzer E., Remold H. G., Welte K., Rubin B. Y., Murray H. W. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984 Aug 1;160(2):600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Hedegaard H. B., Johnston R. B., Jr Cultured human monocytes require exposure to bacterial products to maintain an optimal oxygen radical response. J Immunol. 1982 Jan;128(1):123–128. [PubMed] [Google Scholar]
- Parks D. R., Bryan V. M., Oi V. T., Herzenberg L. A. Antigen-specific identification and cloning of hybridomas with a fluorescence-activated cell sorter. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1962–1966. doi: 10.1073/pnas.76.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Rugtveit J., Brandtzaeg P., Halstensen T. S., Fausa O., Scott H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994 May;35(5):669–674. doi: 10.1136/gut.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan G. W., Carper H. T., Mandell G. L. The effect of three human recombinant hematopoietic growth factors (granulocyte-macrophage colony-stimulating factor, granulocyte colony-stimulating factor, and interleukin-3) on phagocyte oxidative activity. Blood. 1993 Apr 1;81(7):1863–1870. [PubMed] [Google Scholar]
- Szefler S. J., Norton C. E., Ball B., Gross J. M., Aida Y., Pabst M. J. IFN-gamma and LPS overcome glucocorticoid inhibition of priming for superoxide release in human monocytes. Evidence that secretion of IL-1 and tumor necrosis factor-alpha is not essential for monocyte priming. J Immunol. 1989 Jun 1;142(11):3985–3992. [PubMed] [Google Scholar]
- Ulevitch R. J., Tobias P. S. Recognition of endotoxin by cells leading to transmembrane signaling. Curr Opin Immunol. 1994 Feb;6(1):125–130. doi: 10.1016/0952-7915(94)90043-4. [DOI] [PubMed] [Google Scholar]



