Abstract
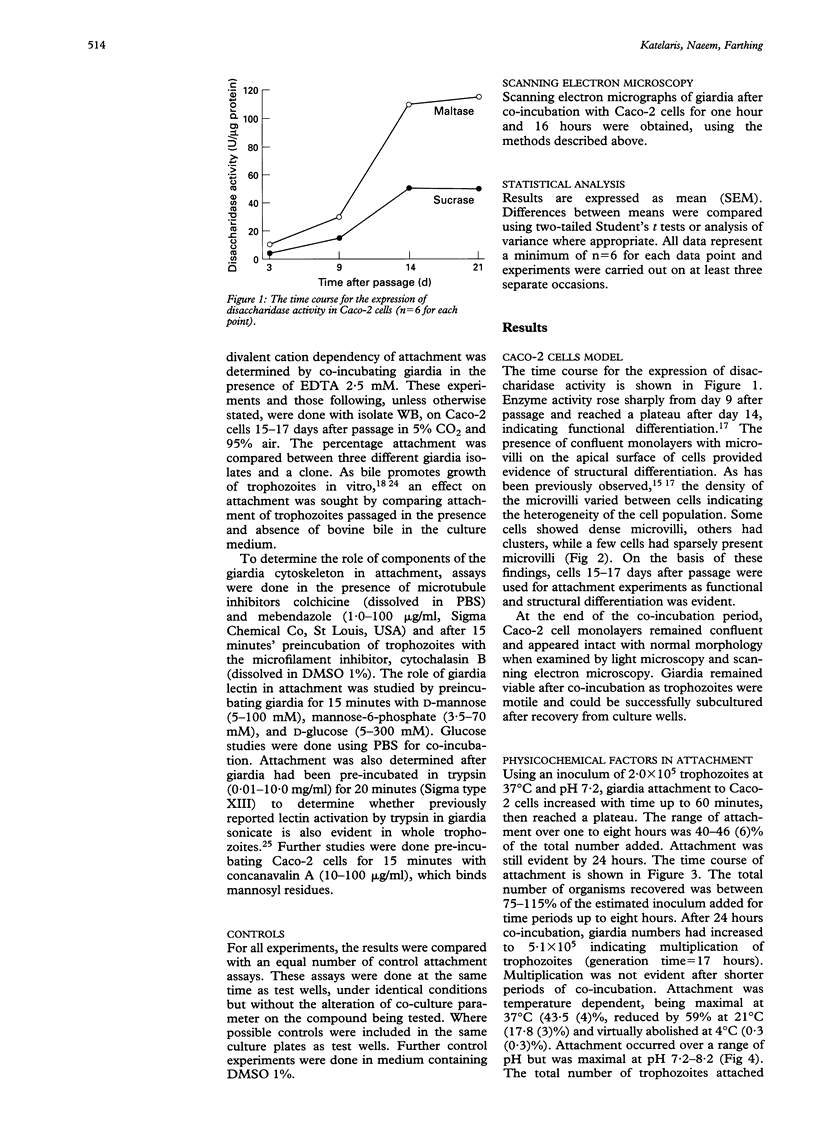
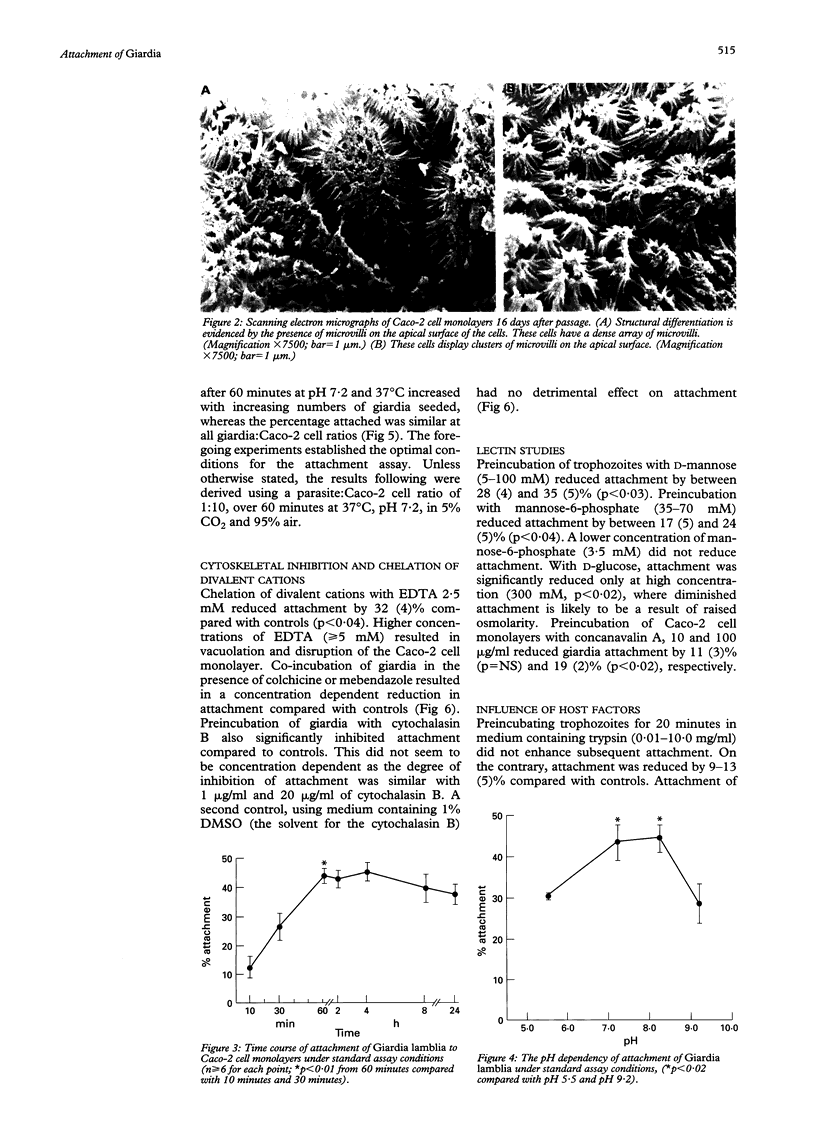
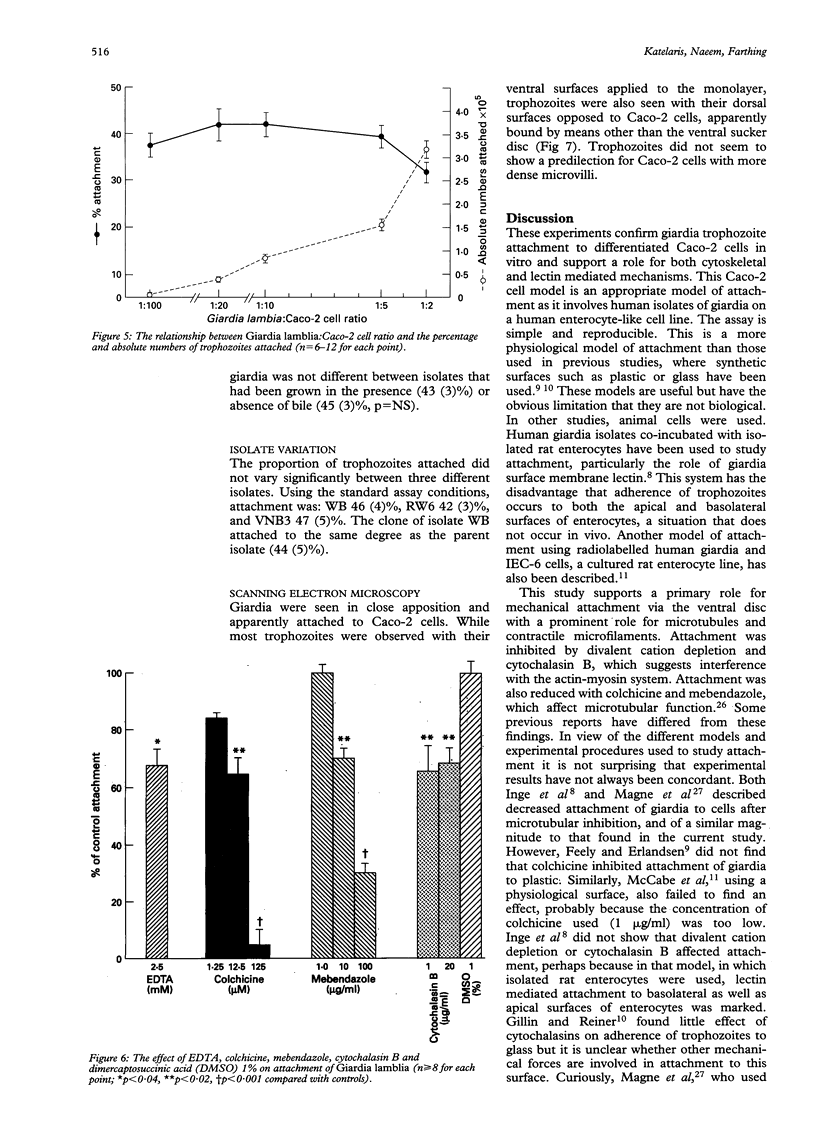
Attachment of Giardia lamblia trophozoites to enterocytes is essential for colonisation of the small intestine and is considered a prerequisite for giardia induced enterocyte damage. The precise mechanisms involved are still being debated and some earlier work has been performed in models of uncertain biological relevance. In this study, co-incubation of giardia with enterocyte-like differentiated Caco-2 cells was used as a model to study the influence of physical and chemical factors on attachment. Giardia attachment was maximal between one and eight hours and stable over pH 7.2-8.2 but it was reduced by acidification. Attachment was dependent on temperature and was maximal at 37 degrees and virtually abolished at 4 degrees C. It was reduced compared with controls (p < 0.05) by EDTA 2.5 mM (mean (SEM) 32 (4)%), colchicine 12.5 microM (35 (5)%), mebendazole 10 micrograms/ml (30 (3)%), and cytochalasin B 1 microgram/ml (34 (3)%). Giardia attachment was also diminished by preincubation with mannose 50 mM or mannose-6-phosphate 35 mM (21 (4); 17 (5)%) or by preincubating Caco-2 cells with concanavalin A 100 micrograms/ml (19 (2)%). Enhanced binding was not evident after trypsinisation of trophozoites. Scanning electron microscopy showed that giardia seemed to attach to the Caco-2 monolayer predominantly by its ventral surface but dorsal orientation was also observed. No difference in attachment was observed between three different giardia isolates or a parent isolate and its clone. Attachment of giardia to Caco-2 cells is primarily by cytoskeletal mechanisms, inhibitable by interference with contractile filaments and microtubules, while attachment by mannose binding lectin also seems to mediate binding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum K. F., Berens R. L., Jones R. H., Marr J. J. A new method for cloning Giardia lamblia, with a discussion of the statistical considerations of limiting dilution. J Parasitol. 1988 Apr;74(2):267–269. [PubMed] [Google Scholar]
- Belosevic M., Faubert G. M., MacLean J. D. Disaccharidase activity in the small intestine of gerbils (Meriones unguiculatus) during primary and challenge infections with Giardia lamblia. Gut. 1989 Sep;30(9):1213–1219. doi: 10.1136/gut.30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968 Jan;22(1):99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- Edlind T. D., Hang T. L., Chakraborty P. R. Activity of the anthelmintic benzimidazoles against Giardia lamblia in vitro. J Infect Dis. 1990 Dec;162(6):1408–1411. doi: 10.1093/infdis/162.6.1408. [DOI] [PubMed] [Google Scholar]
- Farthing M. J., Pereira M. E., Keusch G. T. Description and characterization of a surface lectin from Giardia lamblia. Infect Immun. 1986 Feb;51(2):661–667. doi: 10.1128/iai.51.2.661-667.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farthing M. J., Varon S. R., Keusch G. T. Mammalian bile promotes growth of Giardia lamblia in axenic culture. Trans R Soc Trop Med Hyg. 1983;77(4):467–469. doi: 10.1016/0035-9203(83)90115-3. [DOI] [PubMed] [Google Scholar]
- Favennec L., Chochillon C., Meillet D., Magne D., Savel J., Raichvarg D., Gobert J. G. Adherence and multiplication of Giardia intestinalis on human enterocyte-like differentiated cells in vitro. Parasitol Res. 1990;76(7):581–584. doi: 10.1007/BF00932566. [DOI] [PubMed] [Google Scholar]
- Feely D. E., Erlandsen S. L. Effect of cytochalasin-B, low Ca++ concentration, iodoacetic acid, and quinacrine-HCl on the attachment of Giardia trophozoites in vitro. J Parasitol. 1982 Oct;68(5):869–873. [PubMed] [Google Scholar]
- Feely D. E., Schollmeyer J. V., Erlandsen S. L. Giardia spp.: distribution of contractile proteins in the attachment organelle. Exp Parasitol. 1982 Feb;53(1):145–154. doi: 10.1016/0014-4894(82)90100-x. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Reiner D. S. Attachment of the flagellate Giardia lamblia: role of reducing agents, serum, temperature, and ionic composition. Mol Cell Biol. 1982 Apr;2(4):369–377. doi: 10.1128/mcb.2.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy R. A., Bertrand S., Faubert G. M. Modification of RPMI 1640 for use in vitro immunological studies of host-parasite interactions in giardiasis. J Clin Microbiol. 1991 Mar;29(3):627–629. doi: 10.1128/jcm.29.3.627-629.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo I. J., Raub T. J., Borchardt R. T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989 Mar;96(3):736–749. [PubMed] [Google Scholar]
- Holberton D. V. Arrangement of subunits in microribbons from Giardia. J Cell Sci. 1981 Feb;47:167–185. doi: 10.1242/jcs.47.1.167. [DOI] [PubMed] [Google Scholar]
- Holberton D. V. Attachment of Giardia-a hydrodynamic model based on flagellar activity. J Exp Biol. 1974 Feb;60(1):207–221. doi: 10.1242/jeb.60.1.207. [DOI] [PubMed] [Google Scholar]
- Inge P. M., Edson C. M., Farthing M. J. Attachment of Giardia lamblia to rat intestinal epithelial cells. Gut. 1988 Jun;29(6):795–801. doi: 10.1136/gut.29.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D. B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77(4):487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Lev B., Ward H., Keusch G. T., Pereira M. E. Lectin activation in Giardia lamblia by host protease: a novel host-parasite interaction. Science. 1986 Apr 4;232(4746):71–73. doi: 10.1126/science.3513312. [DOI] [PubMed] [Google Scholar]
- Magne D., Favennec L., Chochillon C., Gorenflot A., Meillet D., Kapel N., Raichvarg D., Savel J., Gobert J. G. Role of cytoskeleton and surface lectins in Giardia duodenalis attachment to Caco2 cells. Parasitol Res. 1991;77(8):659–662. doi: 10.1007/BF00928679. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Erickson R. H., Gum J. R., Yoshioka M., Gum E., Kim Y. S. Biosynthesis of alkaline phosphatase during differentiation of the human colon cancer cell line Caco-2. Gastroenterology. 1990 May;98(5 Pt 1):1199–1207. doi: 10.1016/0016-5085(90)90334-w. [DOI] [PubMed] [Google Scholar]
- Peattie D. A., Alonso R. A., Hein A., Caulfield J. P. Ultrastructural localization of giardins to the edges of disk microribbons of Giarida lamblia and the nucleotide and deduced protein sequence of alpha giardin. J Cell Biol. 1989 Nov;109(5):2323–2335. doi: 10.1083/jcb.109.5.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. D. Pathophysiology and immunology of giardiasis. Annu Rev Med. 1985;36:295–307. doi: 10.1146/annurev.me.36.020185.001455. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Vachon P. H., Beaulieu J. F. Transient mosaic patterns of morphological and functional differentiation in the Caco-2 cell line. Gastroenterology. 1992 Aug;103(2):414–423. doi: 10.1016/0016-5085(92)90829-n. [DOI] [PubMed] [Google Scholar]
- Ward H. D., Lev B. I., Kane A. V., Keusch G. T., Pereira M. E. Identification and characterization of taglin, a mannose 6-phosphate binding, trypsin-activated lectin from Giardia lamblia. Biochemistry. 1987 Dec 29;26(26):8669–8675. doi: 10.1021/bi00400a027. [DOI] [PubMed] [Google Scholar]




