Abstract
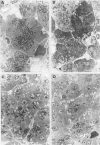
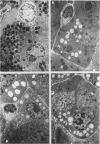
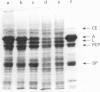
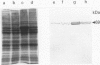
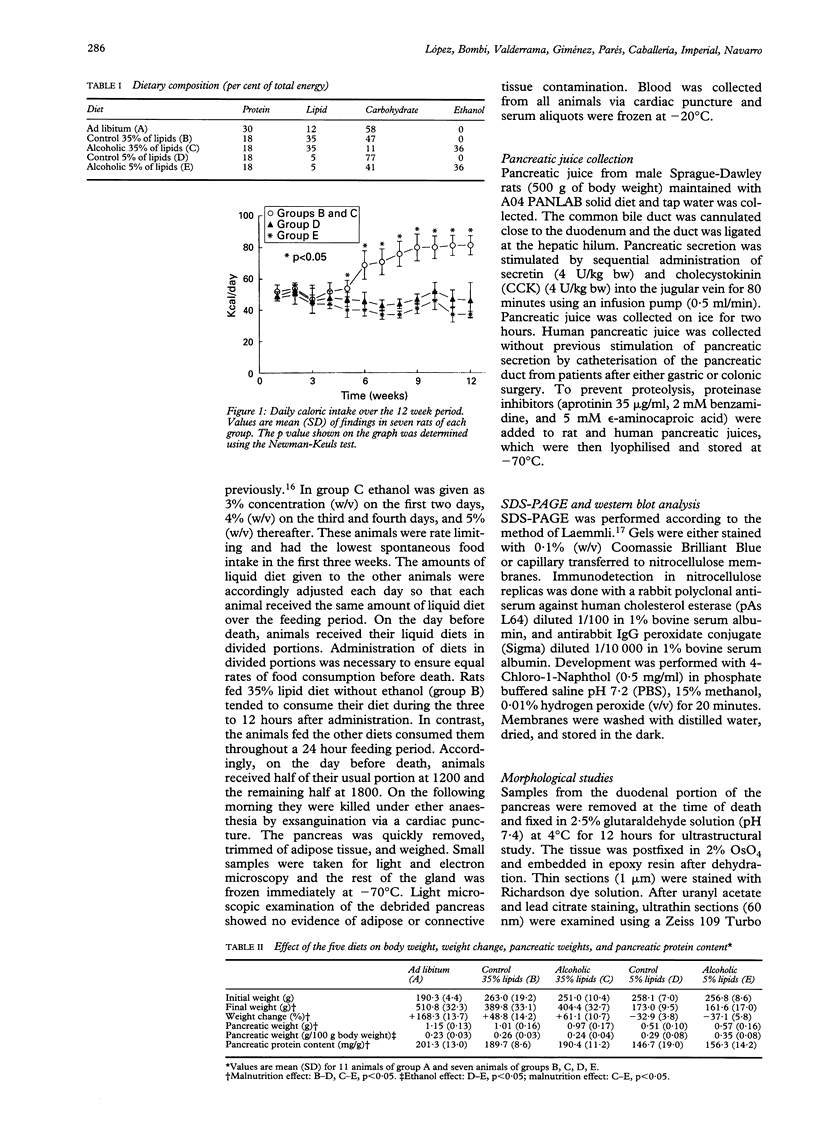
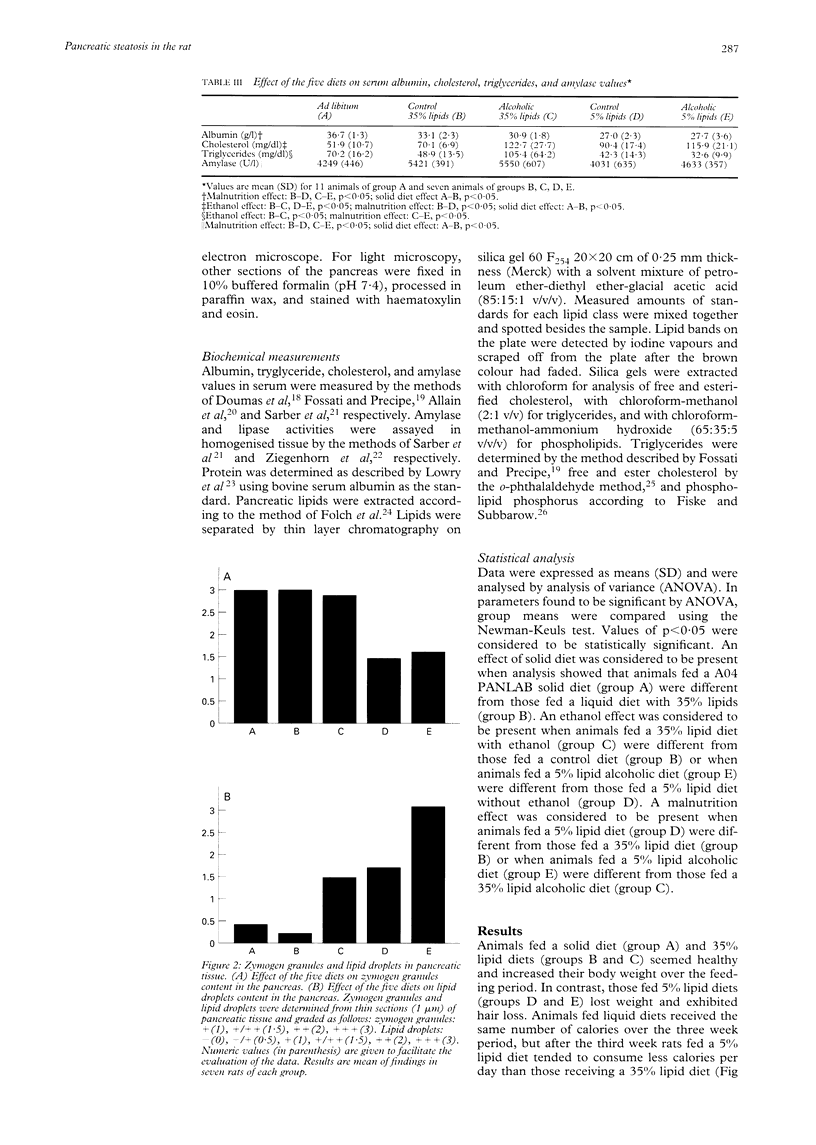
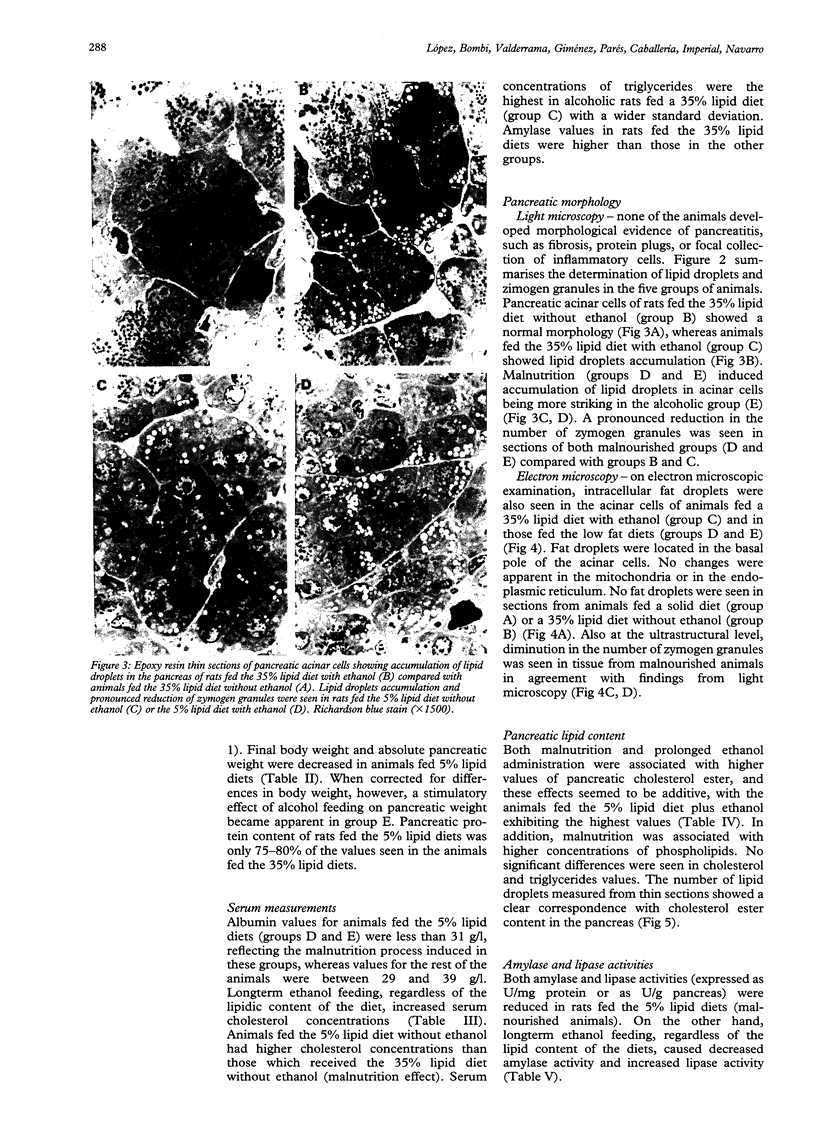
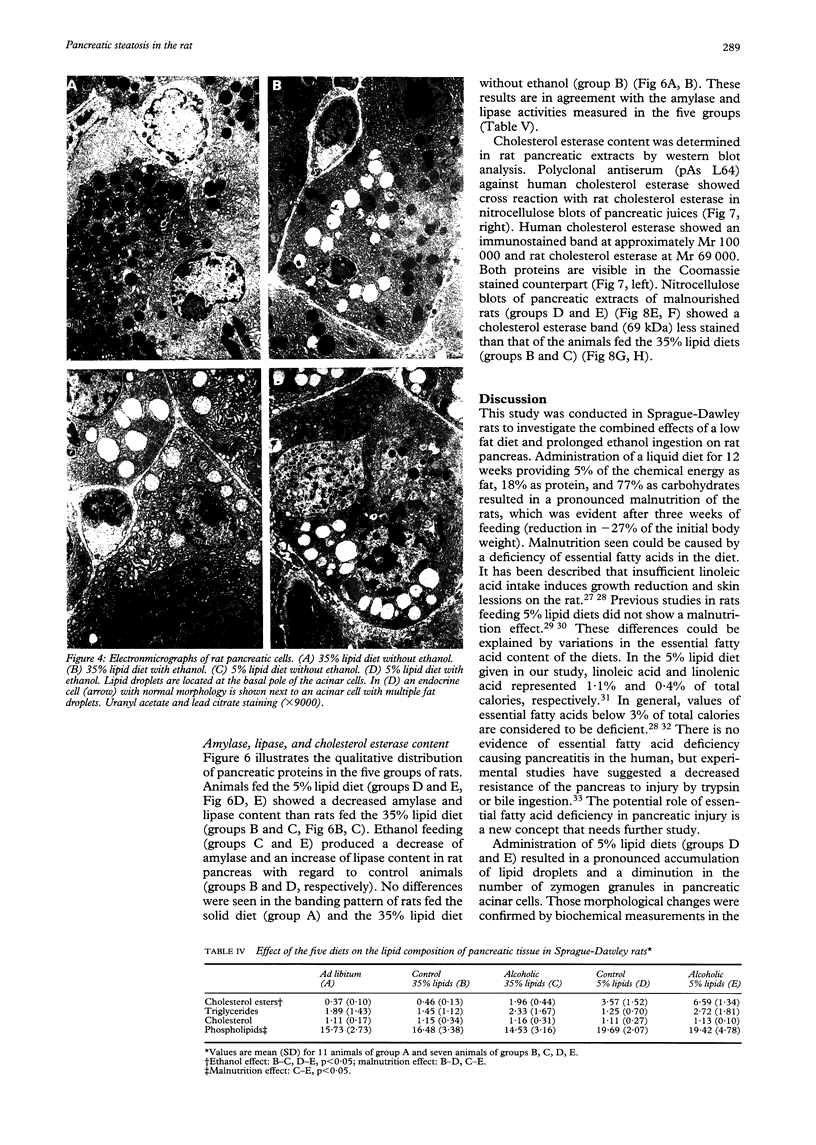
Nutritional factors, especially the protein and fat content of the diet, may change pancreatic morphology after ethanol induced injury. This study was performed to delineate the combined effects of a low fat diet and longterm ethanol ingestion on the rat pancreas. Male Sprague-Dawley rats were maintained with five different diets for 12 weeks and the pancreas removed on the day they were killed. Rats fed a very low fat diet without ethanol (5% of total calories as lipid) developed malnutrition, pancreatic steatosis, and reduction in zymogen granules content. Animals fed a 35% lipid diet with ethanol also developed pancreatic steatosis but changes in zymogen granules content were not detected. Both malnutrition and longterm ethanol consumption increased pancreatic cholesterol ester content, and their effects were additive. Pancreatic steatosis was accompanied with hypercholesterolaemia. Amylase, lipase, and cholesterol esterase content were reduced in malnourished rats; but longterm ethanol ingestion, regardless of the nutritional state, increased lipase content and decreased amylase. It is suggested that high serum cholesterol concentrations and increased pancreatic lipase activity could cause accumulation of cholesterol esters in acinar cells. Fat accumulation in the pancreas has been reported as the earliest histopathological feature in alcoholic patients and may be responsible for cytotoxic effects on the acinar cells at the level of the cell membrane. Although it is difficult to extrapolate results in this animal study to the human situation, the results presented in this work might explain the higher incidence of pancreatitis is malnourished populations as well as in alcoholic subjects that is reported in dietary surveys.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfin-Slater R. B., Aftergood L. Essential fatty acids reinvestigated. Physiol Rev. 1968 Oct;48(4):758–784. doi: 10.1152/physrev.1968.48.4.758. [DOI] [PubMed] [Google Scholar]
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- Alling C., Bruce A., Karlsson I., Svennerholm L. The effect of different dietary levels of essential fatty acids on growth of the rat. Nutr Metab. 1974;16(1):38–50. doi: 10.1159/000175471. [DOI] [PubMed] [Google Scholar]
- Balakrishnan V., Sauniere J. F., Hariharan M., Sarles H. Diet, pancreatic function, and chronic pancreatitis in south India and France. Pancreas. 1988;3(1):30–35. doi: 10.1097/00006676-198802000-00006. [DOI] [PubMed] [Google Scholar]
- Berlin R. M. Insomnia in hospitalized patients: approaches to management. Psychiatr Med. 1986;4(2):197–208. [PubMed] [Google Scholar]
- Chang C. C., Chang T. Y. Cycloheximide sensitivity in regulation of acyl coenzyme A:cholesterol acyltransferase activity in Chinese hamster ovary cells. 2. Effect of sterol endogenously synthesized. Biochemistry. 1986 Apr 8;25(7):1700–1706. doi: 10.1021/bi00355a039. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Huh H. Y., Cadigan K. M., Chang T. Y. Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J Biol Chem. 1993 Oct 5;268(28):20747–20755. [PubMed] [Google Scholar]
- Doumas B. T., Watson W. A., Biggs H. G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971 Jan;31(1):87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- Forrest B. J., Cushley R. J. Cholesterol esters and membrane permeability. A nuclear magnetic resonance (MNR) study. Atherosclerosis. 1977 Nov;28(3):309–318. doi: 10.1016/0021-9150(77)90178-2. [DOI] [PubMed] [Google Scholar]
- Fossati P., Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982 Oct;28(10):2077–2080. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Hamamoto T., Yamada S., Hirayama C. Nonoxidative metabolism of ethanol in the pancreas; implication in alcoholic pancreatic damage. Biochem Pharmacol. 1990 Jan 15;39(2):241–245. doi: 10.1016/0006-2952(90)90022-d. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laposata E. A., Harrison E. H., Hedberg E. B. Synthesis and degradation of fatty acid ethyl esters by cultured hepatoma cells exposed to ethanol. J Biol Chem. 1990 Jun 15;265(17):9688–9693. [PubMed] [Google Scholar]
- Laposata E. A., Lange L. G. Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science. 1986 Jan 31;231(4737):497–499. doi: 10.1126/science.3941913. [DOI] [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. The feeding of ethanol in liquid diets. Alcohol Clin Exp Res. 1986 Oct;10(5):550–553. doi: 10.1111/j.1530-0277.1986.tb05140.x. [DOI] [PubMed] [Google Scholar]
- Lieber C. S., Lasker J. M., DeCarli L. M., Saeli J., Wojtowicz T. Role of acetone, dietary fat and total energy intake in induction of hepatic microsomal ethanol oxidizing system. J Pharmacol Exp Ther. 1988 Nov;247(2):791–795. [PubMed] [Google Scholar]
- Manautou J. E., Carlson G. P. Ethanol-induced fatty acid ethyl ester formation in vivo and in vitro in rat lung. Toxicology. 1991;70(3):303–312. doi: 10.1016/0300-483x(91)90005-l. [DOI] [PubMed] [Google Scholar]
- Montalegre A., Sarles H., Ricosse J. H., Sahel J. Pancreatic lesions due to prolonged malnutrition in Ibo children: possible transition between kwashiorkor and chronic calcifying pancreatitis. Pancreas. 1987;2(1):114–116. doi: 10.1097/00006676-198701000-00017. [DOI] [PubMed] [Google Scholar]
- Pitchumoni C. S. Pancreas in primary malnutrition disorders. Am J Clin Nutr. 1973 Mar;26(3):374–379. doi: 10.1093/ajcn/26.3.374. [DOI] [PubMed] [Google Scholar]
- Pitchumoni C. S., Sonnenshein M., Candido F. M., Panchacharam P., Cooperman J. M. Nutrition in the pathogenesis of alcoholic pancreatitis. Am J Clin Nutr. 1980 Mar;33(3):631–636. doi: 10.1093/ajcn/33.3.631. [DOI] [PubMed] [Google Scholar]
- Pitchumoni C. S., Thomas E. Letter: Chronic cassava toxicity: possible relationship to chronic pancreatic disease in malnourished populations. Lancet. 1973 Dec 15;2(7842):1397–1398. doi: 10.1016/s0140-6736(73)93377-1. [DOI] [PubMed] [Google Scholar]
- Ponnappa B. C., Hoek J. B., Sarchet K., Rubin E. Dietary carbohydrate level determines the effect of long-term ethanol ingestion on rat pancreatic amylase content. J Lab Clin Med. 1986 Jun;107(6):556–562. [PubMed] [Google Scholar]
- Rafael J., Patzelt J., Elmadfa I. Effect of dietary linoleic acid and essential fatty acid deficiency on resting metabolism, nonshivering thermogenesis and brown adipose tissue in the rat. J Nutr. 1988 May;118(5):627–632. doi: 10.1093/jn/118.5.627. [DOI] [PubMed] [Google Scholar]
- Riley D. J., Kyger E. M., Spilburg C. A., Lange L. G. Pancreatic cholesterol esterases. 2. Purification and characterization of human pancreatic fatty acid ethyl ester synthase. Biochemistry. 1990 Apr 24;29(16):3848–3852. doi: 10.1021/bi00468a007. [DOI] [PubMed] [Google Scholar]
- Rudel L. L., Morris M. D. Determination of cholesterol using o-phthalaldehyde. J Lipid Res. 1973 May;14(3):364–366. [PubMed] [Google Scholar]
- Sankaran H., Deveney C. W., Larkin E. C., Rao G. A. Carbohydrate intake determines pancreatic acinar amylase activity and release despite chronic alcoholemia in rats. J Nutr. 1992 Sep;122(9):1884–1891. doi: 10.1093/jn/122.9.1884. [DOI] [PubMed] [Google Scholar]
- Sarles H. An international survey on nutrition and pancreatitis. Digestion. 1973;9(5):389–403. doi: 10.1159/000197468. [DOI] [PubMed] [Google Scholar]
- Sarles H., Cros R. C., Bidart J. M. A multicenter inquiry into the etiology of pancreatic diseases. Digestion. 1979;19(2):110–125. doi: 10.1159/000198331. [DOI] [PubMed] [Google Scholar]
- Simsek H., Singh M. Effect of prolonged ethanol intake on pancreatic lipids in the rat pancreas. Pancreas. 1990 Jul;5(4):401–407. doi: 10.1097/00006676-199007000-00005. [DOI] [PubMed] [Google Scholar]
- Tasso F., Clop J., Sarles H. The interaction of ethanol, dietary lipids and proteins on the rat pancreas. II. Ultrastructural study. Digestion. 1971;4(1):23–34. doi: 10.1159/000197093. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H., Towner S. J., Yu G. S., French S. W. Potentiation of ethanol-induced pancreatic injury by dietary fat. Induction of chronic pancreatitis by alcohol in rats. Am J Pathol. 1988 May;131(2):246–257. [PMC free article] [PubMed] [Google Scholar]
- WEISBLUM B., HERMAN L., FITZGERALD P. J. Changes in pancreatic acinar cells during protein deprivation. J Cell Biol. 1962 Feb;12:313–327. doi: 10.1083/jcb.12.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. S., Colley P. W., Sosula L., Pirola R. C., Chapman B. A., Somer J. B. Alcohol causes a fatty pancreas. A rat model of ethanol-induced pancreatic steatosis. Alcohol Clin Exp Res. 1982 Winter;6(1):117–121. doi: 10.1111/j.1530-0277.1982.tb05389.x. [DOI] [PubMed] [Google Scholar]
- Wilson J. S., Korsten M. A., Leo M. A., Lieber C. S. Combined effects of protein deficiency and chronic ethanol consumption on rat pancreas. Dig Dis Sci. 1988 Oct;33(10):1250–1259. doi: 10.1007/BF01536675. [DOI] [PubMed] [Google Scholar]
- Wilson J. S., Somer J. B., Pirola R. C. Chronic ethanol feeding causes accumulation of serum cholesterol in rat pancreas. Exp Mol Pathol. 1984 Dec;41(3):289–297. doi: 10.1016/0014-4800(84)90016-9. [DOI] [PubMed] [Google Scholar]