Abstract
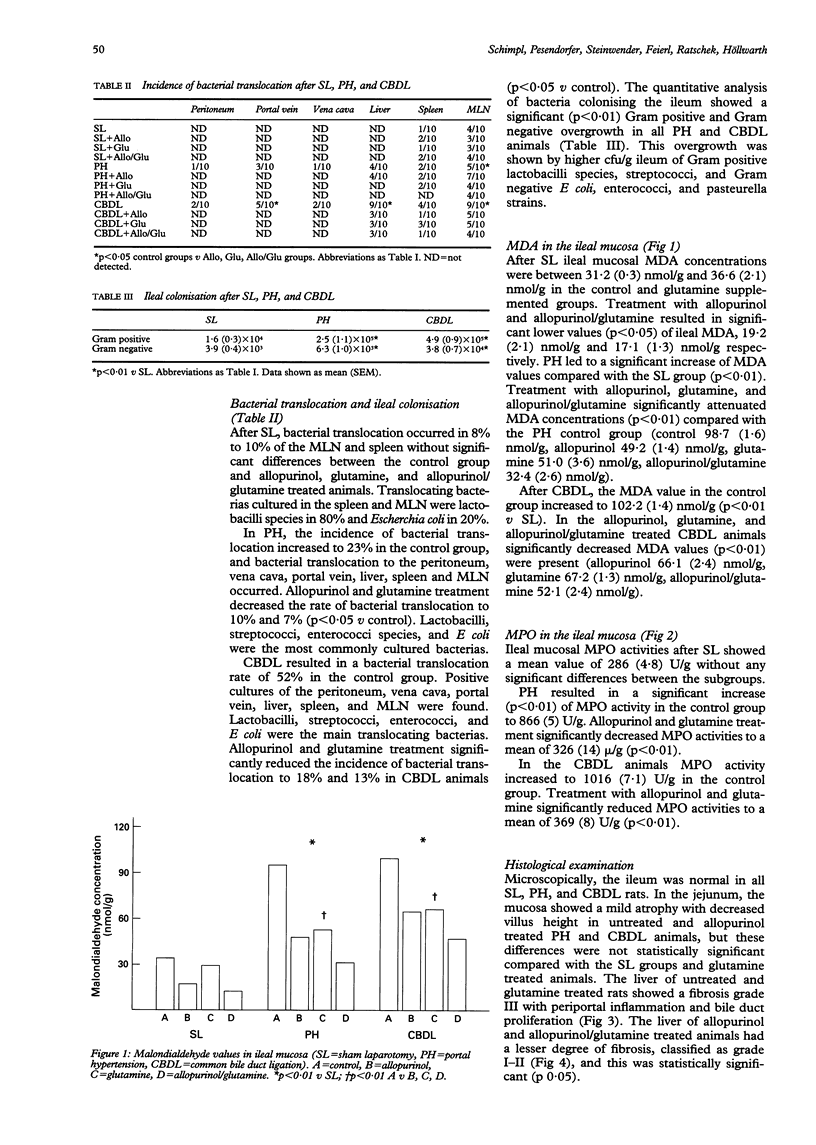
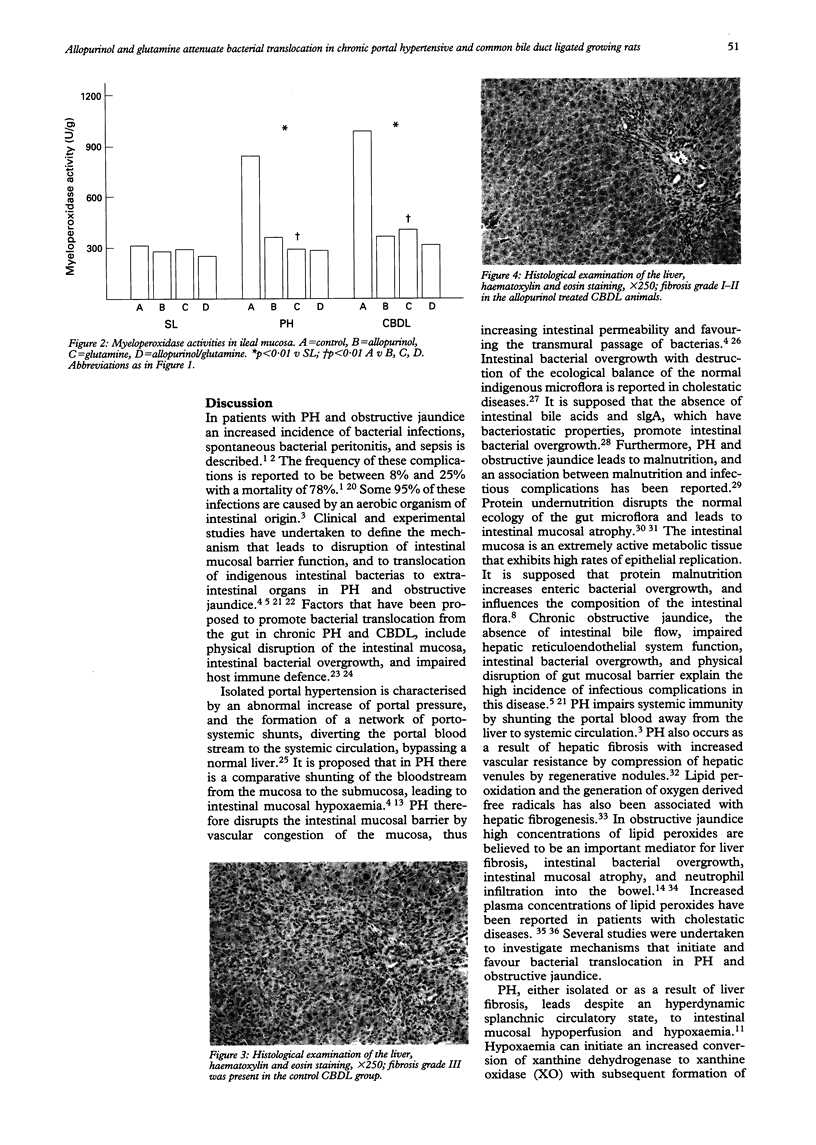
BACKGROUND: Spontaneous bacterial infections and septicaemia result in morbidity and mortality in patients with portal hypertension and obstructive jaundice. AIM: The aim of this study in rats was to investigate the incidence of bacterial translocation in portal hypertension and obstructive jaundice, and to evaluate the effects of allopurinol and glutamine. METHODS: Rats were subjected to sham laparotomy (SL), portal hypertension (PH) by calibrated stenosis of the portal vein, and common bile duct ligation (CBDL). Animals of each group were either treated with allopurinol (50 mg/kg twice a week), glutamine (1 g/kg/d), and allopurinol and glutamine. RESULTS: After four weeks, significant bacterial translocation in the untreated PH and CBDL rats occurred. Intestinal mucosal malondialdehyde concentrations (MDA), as an indicator for lipid peroxidation, and myeloperoxidase activity (MPO) released from activated neutrophils were also significantly increased (p < 0.01). Allopurinol and glutamine in PH and CBDL rats improved bacterial translocation, and decreased MDA and MPO values (p < 0.01). CONCLUSION: In PH and CBDL rats significant bacterial translocation, ileal mucosal lipid peroxidation, and neutrophil derived MPO activity occurred. Allopurinol and glutamine significantly reduced bacterial translocation, as well as ileal mucosal MDA and MPO activities.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Boyce S. T., Babcock G. F., Gianotti L., Peck M. D., Dunn D. L., Pyles T., Childress C. P., Ash S. K. The process of microbial translocation. Ann Surg. 1990 Oct;212(4):496–512. doi: 10.1097/00000658-199010000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A. E., Jones W. G., 2nd, Minei J. P., Fahey T. J., 3rd, Lowry S. F., Shires G. T. Bacterial overgrowth and intestinal atrophy in the etiology of gut barrier failure in the rat. Am J Surg. 1991 Feb;161(2):300–304. doi: 10.1016/0002-9610(91)91148-c. [DOI] [PubMed] [Google Scholar]
- Barber A. E., Jones W. G., 2nd, Minei J. P., Fahey T. J., 3rd, Moldawer L. L., Rayburn J. L., Fischer E., Keogh C. V., Shires G. T., Lowry S. F. Harry M. Vars award. Glutamine or fiber supplementation of a defined formula diet: impact on bacterial translocation, tissue composition, and response to endotoxin. JPEN J Parenter Enteral Nutr. 1990 Jul-Aug;14(4):335–343. doi: 10.1177/0148607190014004335. [DOI] [PubMed] [Google Scholar]
- Basista M. H., Stauber R. E., Van Thiel D. H., Tauxe W. N., Dindzans V. J. Effect of isolated portal hypertension on Kupffer cell function. Dig Dis Sci. 1994 Jan;39(1):46–50. doi: 10.1007/BF02090059. [DOI] [PubMed] [Google Scholar]
- Blanchet L., Lebrec D. Changes in splanchnic blood flow in portal hypertensive rats. Eur J Clin Invest. 1982 Aug;12(4):327–330. doi: 10.1111/j.1365-2362.1982.tb02240.x. [DOI] [PubMed] [Google Scholar]
- Britton R. S., Bacon B. R. Role of free radicals in liver diseases and hepatic fibrosis. Hepatogastroenterology. 1994 Aug;41(4):343–348. [PubMed] [Google Scholar]
- Cahill C. J., Pain J. A., Bailey M. E. Bile salts, endotoxin and renal function in obstructive jaundice. Surg Gynecol Obstet. 1987 Dec;165(6):519–522. [PubMed] [Google Scholar]
- Dargel R. Lipid peroxidation--a common pathogenetic mechanism? Exp Toxicol Pathol. 1992 Aug;44(4):169–181. doi: 10.1016/S0940-2993(11)80202-2. [DOI] [PubMed] [Google Scholar]
- Deitch E. A. Bacterial translocation: the influence of dietary variables. Gut. 1994 Jan;35(1 Suppl):S23–S27. doi: 10.1136/gut.35.1_suppl.s23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch E. A., Bridges W., Baker J., Ma J. W., Ma L., Grisham M. B., Granger D. N., Specian R. D., Berg R. Hemorrhagic shock-induced bacterial translocation is reduced by xanthine oxidase inhibition or inactivation. Surgery. 1988 Aug;104(2):191–198. [PubMed] [Google Scholar]
- Deitch E. A., Ma W. J., Ma L., Berg R., Specian R. D. Endotoxin-induced bacterial translocation: a study of mechanisms. Surgery. 1989 Aug;106(2):292–300. [PubMed] [Google Scholar]
- Deitch E. A., Sittig K., Li M., Berg R., Specian R. D. Obstructive jaundice promotes bacterial translocation from the gut. Am J Surg. 1990 Jan;159(1):79–84. doi: 10.1016/s0002-9610(05)80610-5. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Taylor M., Grisham M., Ma L., Bridges W., Berg R. Endotoxin induces bacterial translocation and increases xanthine oxidase activity. J Trauma. 1989 Dec;29(12):1679–1683. doi: 10.1097/00005373-198912000-00017. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Taylor M., Grisham M., Ma L., Bridges W., Berg R. Endotoxin induces bacterial translocation and increases xanthine oxidase activity. J Trauma. 1989 Dec;29(12):1679–1683. doi: 10.1097/00005373-198912000-00017. [DOI] [PubMed] [Google Scholar]
- Deitch E. A. The role of intestinal barrier failure and bacterial translocation in the development of systemic infection and multiple organ failure. Arch Surg. 1990 Mar;125(3):403–404. doi: 10.1001/archsurg.1990.01410150125024. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Winterton J., Li M., Berg R. The gut as a portal of entry for bacteremia. Role of protein malnutrition. Ann Surg. 1987 Jun;205(6):681–692. doi: 10.1097/00000658-198706000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J. W., Andersson R., Soltesz V., Willén R., Bengmark S. The role of bile and bile acids in bacterial translocation in obstructive jaundice in rats. Eur Surg Res. 1993 Jan-Feb;25(1):11–19. doi: 10.1159/000129252. [DOI] [PubMed] [Google Scholar]
- Garcia-Tsao G., Albillos A., Barden G. E., West A. B. Bacterial translocation in acute and chronic portal hypertension. Hepatology. 1993 Jun;17(6):1081–1085. [PubMed] [Google Scholar]
- Garcia-Tsao G. Spontaneous bacterial peritonitis. Gastroenterol Clin North Am. 1992 Mar;21(1):257–275. [PubMed] [Google Scholar]
- Gilbert J. A., Kamath P. S. Spontaneous bacterial peritonitis: an update. Mayo Clin Proc. 1995 Apr;70(4):365–370. doi: 10.4065/70.4.365. [DOI] [PubMed] [Google Scholar]
- Krawisz J. E., Sharon P., Stenson W. F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984 Dec;87(6):1344–1350. [PubMed] [Google Scholar]
- Lemonnier F., Cresteil D., Fénéant M., Couturier M., Bernard O., Alagille D. Plasma lipid peroxides in cholestatic children. Acta Paediatr Scand. 1987 Nov;76(6):928–934. doi: 10.1111/j.1651-2227.1987.tb17266.x. [DOI] [PubMed] [Google Scholar]
- Li M., Specian R. D., Berg R. D., Deitch E. A. Effects of protein malnutrition and endotoxin on the intestinal mucosal barrier to the translocation of indigenous flora in mice. JPEN J Parenter Enteral Nutr. 1989 Nov-Dec;13(6):572–578. doi: 10.1177/0148607189013006572. [DOI] [PubMed] [Google Scholar]
- Llovet J. M., Bartolí R., Planas R., Cabré E., Jimenez M., Urban A., Ojanguren I., Arnal J., Gassull M. A. Bacterial translocation in cirrhotic rats. Its role in the development of spontaneous bacterial peritonitis. Gut. 1994 Nov;35(11):1648–1652. doi: 10.1136/gut.35.11.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megison S. M., Horton J. W., Chao H., Walker P. B. Prolonged survival and decreased mucosal injury after low-dose enteral allopurinol prophylaxis in mesenteric ischemia. J Pediatr Surg. 1990 Aug;25(8):917–921. doi: 10.1016/0022-3468(90)90204-m. [DOI] [PubMed] [Google Scholar]
- Mihas A. A., Toussaint J., Hsu H. S., Dotherow P., Achord J. L. Spontaneous bacterial peritonitis in cirrhosis: clinical and laboratory features, survival and prognostic indicators. Hepatogastroenterology. 1992 Dec;39(6):520–522. [PubMed] [Google Scholar]
- Muriel P., Suarez O. R. Role of lipid peroxidation in biliary obstruction in the rat. J Appl Toxicol. 1994 Nov-Dec;14(6):423–426. doi: 10.1002/jat.2550140607. [DOI] [PubMed] [Google Scholar]
- Otamiri T. Oxygen radicals, lipid peroxidation, and neutrophil infiltration after small-intestinal ischemia and reperfusion. Surgery. 1989 May;105(5):593–597. [PubMed] [Google Scholar]
- Runyon B. A. Bacterial infections in patients with cirrhosis. J Hepatol. 1993 Jul;18(3):271–272. doi: 10.1016/s0168-8278(05)80267-3. [DOI] [PubMed] [Google Scholar]
- Sedman P. C., Macfie J., Sagar P., Mitchell C. J., May J., Mancey-Jones B., Johnstone D. The prevalence of gut translocation in humans. Gastroenterology. 1994 Sep;107(3):643–649. doi: 10.1016/0016-5085(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Shibayama Y., Nakata K. Haemodynamic alterations and their morphological basis in biliary obstruction. Liver. 1992 Aug;12(4 Pt 1):175–178. doi: 10.1111/j.1600-0676.1992.tb01043.x. [DOI] [PubMed] [Google Scholar]
- Sorell W. T., Quigley E. M., Jin G., Johnson T. J., Rikkers L. F. Bacterial translocation in the portal-hypertensive rat: studies in basal conditions and on exposure to hemorrhagic shock. Gastroenterology. 1993 Jun;104(6):1722–1726. doi: 10.1016/0016-5085(93)90651-r. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Wirén M., Magnusson K. E., Larsson J. Enteral glutamine increases growth and absorptive capacity of intestinal mucosa in the malnourished rat. Scand J Gastroenterol. 1995 Feb;30(2):146–152. doi: 10.3109/00365529509093253. [DOI] [PubMed] [Google Scholar]
- Wong S. H., Knight J. A., Hopfer S. M., Zaharia O., Leach C. N., Jr, Sunderman F. W., Jr Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin Chem. 1987 Feb;33(2 Pt 1):214–220. [PubMed] [Google Scholar]
- al Amri S. M., Allam A. R., al Mofleh I. A. Spontaneous bacterial peritonitis and culture negative neutrocytic ascites in patients with non-alcoholic liver cirrhosis. J Gastroenterol Hepatol. 1994 Sep-Oct;9(5):433–436. doi: 10.1111/j.1440-1746.1994.tb01269.x. [DOI] [PubMed] [Google Scholar]
- de Groot H. Reactive oxygen species in tissue injury. Hepatogastroenterology. 1994 Aug;41(4):328–332. [PubMed] [Google Scholar]
- van der Hulst R. R., van Kreel B. K., von Meyenfeldt M. F., Brummer R. J., Arends J. W., Deutz N. E., Soeters P. B. Glutamine and the preservation of gut integrity. Lancet. 1993 May 29;341(8857):1363–1365. doi: 10.1016/0140-6736(93)90939-e. [DOI] [PubMed] [Google Scholar]




