Abstract
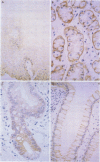
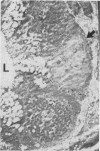
BACKGROUND: While it is clear that luminal epidermal growth factor (EGF) stimulates repair of the damaged bowel, its significance in maintaining normal gut growth remains uncertain. If EGF is important in maintaining normal gut growth, the EGF receptor (EGF-R) should be present on the apical (luminal) surface in addition to the basolateral surface. AIMS/SUBJECTS/METHODS: This study examined the distribution of the EGF-R in the epithelium throughout the human gastro-intestinal tract using immunohistochemistry, electron microscopy, and western blotting of brush border preparations. RESULTS: Immunostaining of the oesophagus showed circumferential EGF-R positivity in the cells of the basal portions of the stratified squamous epithelium but surface cells were EGF-R negative. In the normal stomach, small intestine, and colon, immunostaining localised the receptor to the basolateral surface with the apical membranes being consistently negative. EGF-R positivity within the small intestine appeared to be almost entirely restricted to the proliferative (crypt) region. Western blotting demonstrated a 170 kDa protein in whole tissue homogenates but not in the brush border vesicle preparations. CONCLUSIONS: As the EGF-R is located only on the basolateral surfaces in the normal adult gastrointestinal tract, the major role of luminal EGF is probably to stimulate repair rather than to maintain normal gut growth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goodlad R. A., Wilson T. J., Lenton W., Gregory H., McCullagh K. G., Wright N. A. Intravenous but not intragastric urogastrone-EGF is trophic to the intestine of parenterally fed rats. Gut. 1987 May;28(5):573–582. doi: 10.1136/gut.28.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz P. U., Kasper M., van Noorden S., Polak J. M., Gregory H., Pearse A. G. Immunohistochemical localisation of urogastrone to human duodenal and submandibular glands. Gut. 1978 May;19(5):408–413. doi: 10.1136/gut.19.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormi K., Lehy T. Developmental expression of transforming growth factor-alpha and epidermal growth factor receptor proteins in the human pancreas and digestive tract. Cell Tissue Res. 1994 Dec;278(3):439–450. doi: 10.1007/BF00331362. [DOI] [PubMed] [Google Scholar]
- Jankowski J., Murphy S., Coghill G., Grant A., Wormsley K. G., Sanders D. S., Kerr M., Hopwood D. Epidermal growth factor receptors in the oesophagus. Gut. 1992 Apr;33(4):439–443. doi: 10.1136/gut.33.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski J., al-Rawi H. J., Johnston D. A., Hopwood D., Filipe M. I., Coghill G., Wormsley K. G. Growth regulatory peptides in gastric mucosa. Clin Sci (Lond) 1992 May;82(5):581–587. doi: 10.1042/cs0820581. [DOI] [PubMed] [Google Scholar]
- Kelly D., McFadyen M., King T. P., Morgan P. J. Characterization and autoradiographic localization of the epidermal growth factor receptor in the jejunum of neonatal and weaned pigs. Reprod Fertil Dev. 1992;4(2):183–191. doi: 10.1071/rd9920183. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Mori S., Morishita Y., Sakai K., Kurimoto S., Okamoto M., Kawamoto T., Kuroki T. Electron microscopic evidence for epidermal growth factor receptor (EGF-R)-like immunoreactivity associated with the basolateral surface of gastric parietal cells. Acta Pathol Jpn. 1987 Dec;37(12):1909–1917. doi: 10.1111/j.1440-1827.1987.tb03305.x. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Marchbank T., Calnan D. P., Calam J., Royston P., Batten J. J., Hansen H. F. Epidermal growth factor is digested to smaller, less active forms in acidic gastric juice. Gastroenterology. 1995 Jan;108(1):92–101. doi: 10.1016/0016-5085(95)90012-8. [DOI] [PubMed] [Google Scholar]
- Playford R. J. Peptides and gastrointestinal mucosal integrity. Gut. 1995 Nov;37(5):595–597. doi: 10.1136/gut.37.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford R. J., Woodman A. C., Clark P., Watanapa P., Vesey D., Deprez P. H., Williamson R. C., Calam J. Effect of luminal growth factor preservation on intestinal growth. Lancet. 1993 Apr 3;341(8849):843–848. doi: 10.1016/0140-6736(93)93057-8. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Peters T. J. Studies of Fe3+ transport across isolated intestinal brush-border membrane of the mouse. Biochim Biophys Acta. 1984 May 16;772(2):220–226. doi: 10.1016/0005-2736(84)90047-6. [DOI] [PubMed] [Google Scholar]
- Thompson J. F., van den Berg M., Stokkers P. C. Developmental regulation of epidermal growth factor receptor kinase in rat intestine. Gastroenterology. 1994 Nov;107(5):1278–1287. doi: 10.1016/0016-5085(94)90528-2. [DOI] [PubMed] [Google Scholar]