Abstract
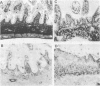
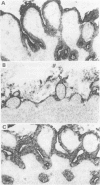
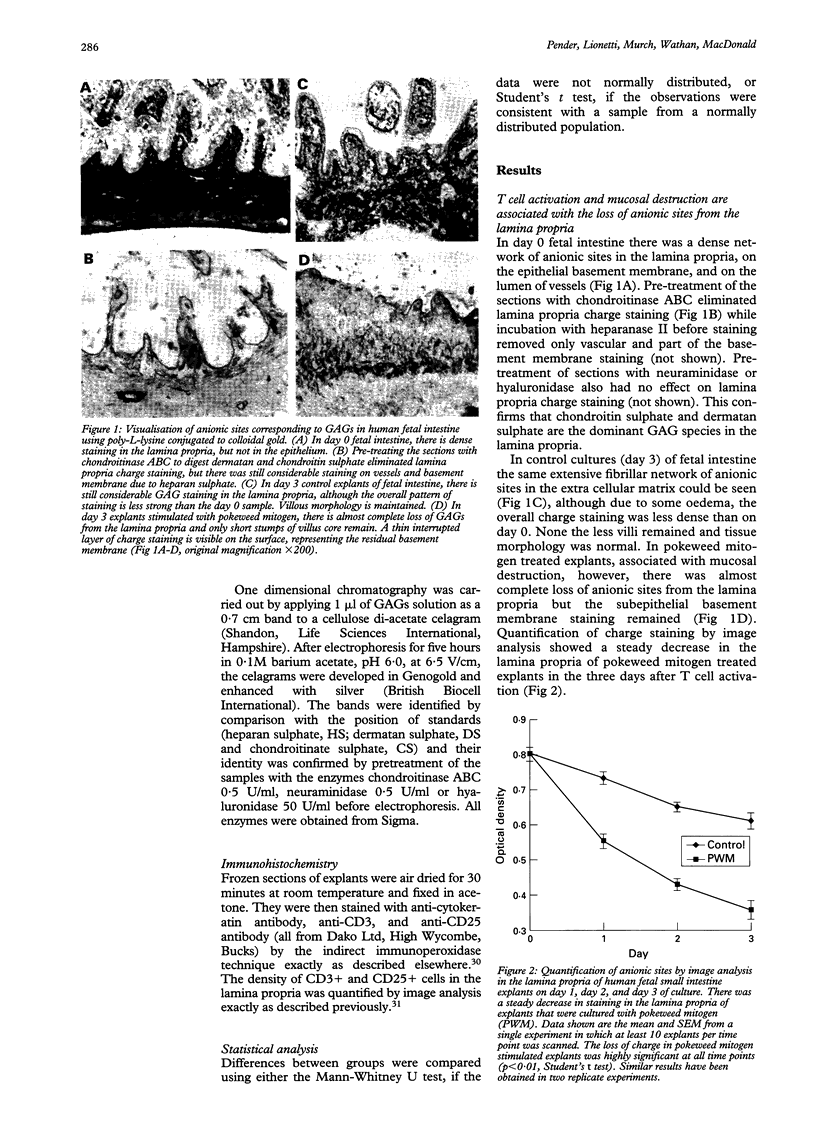
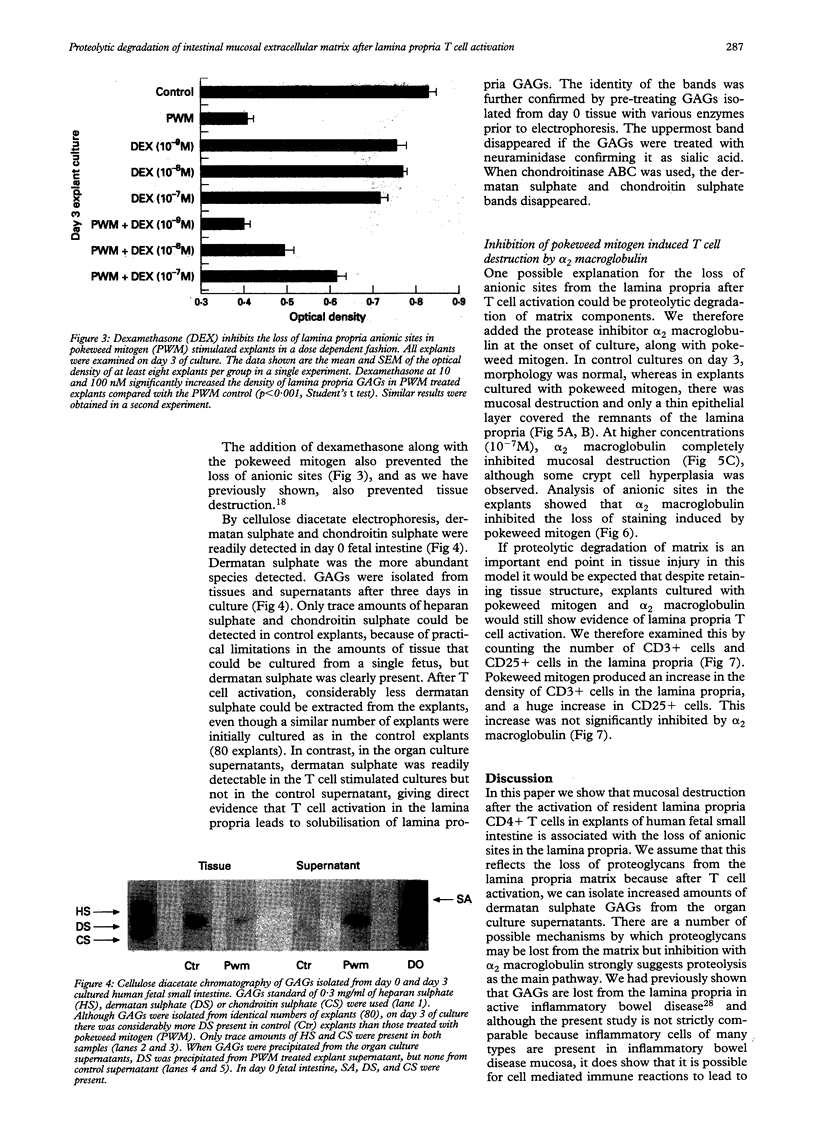
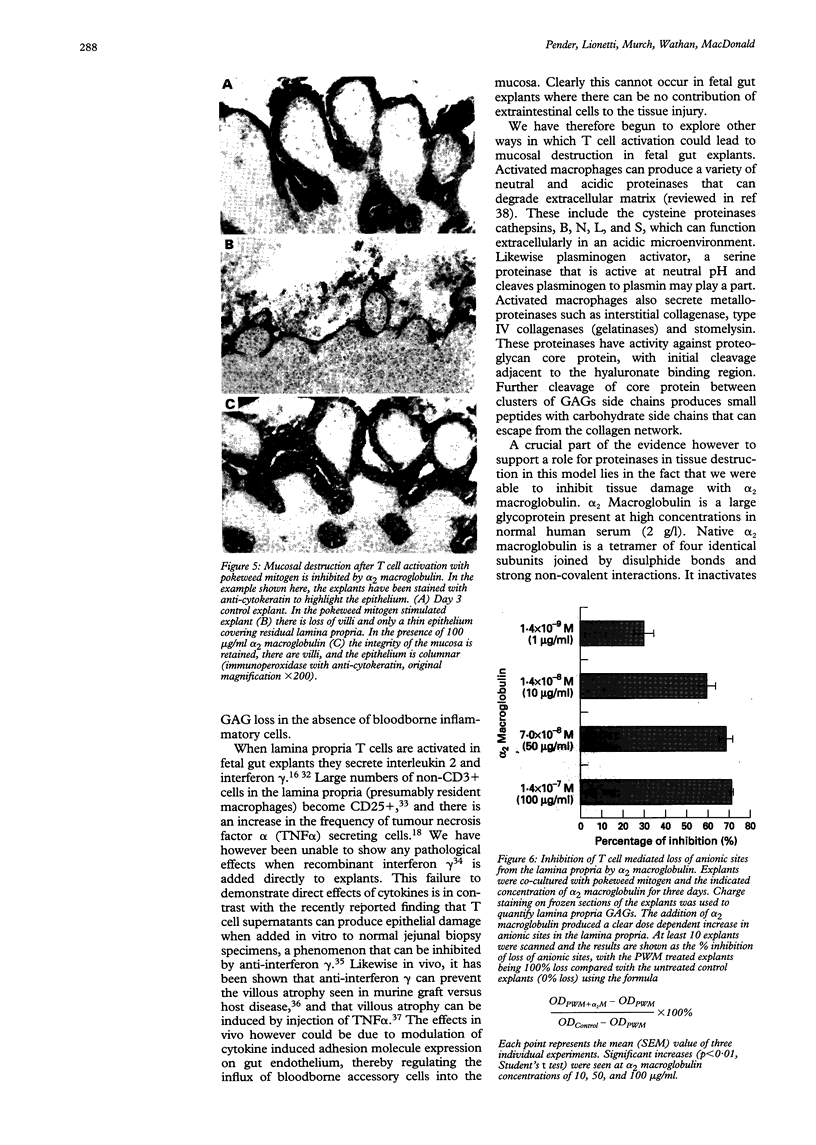
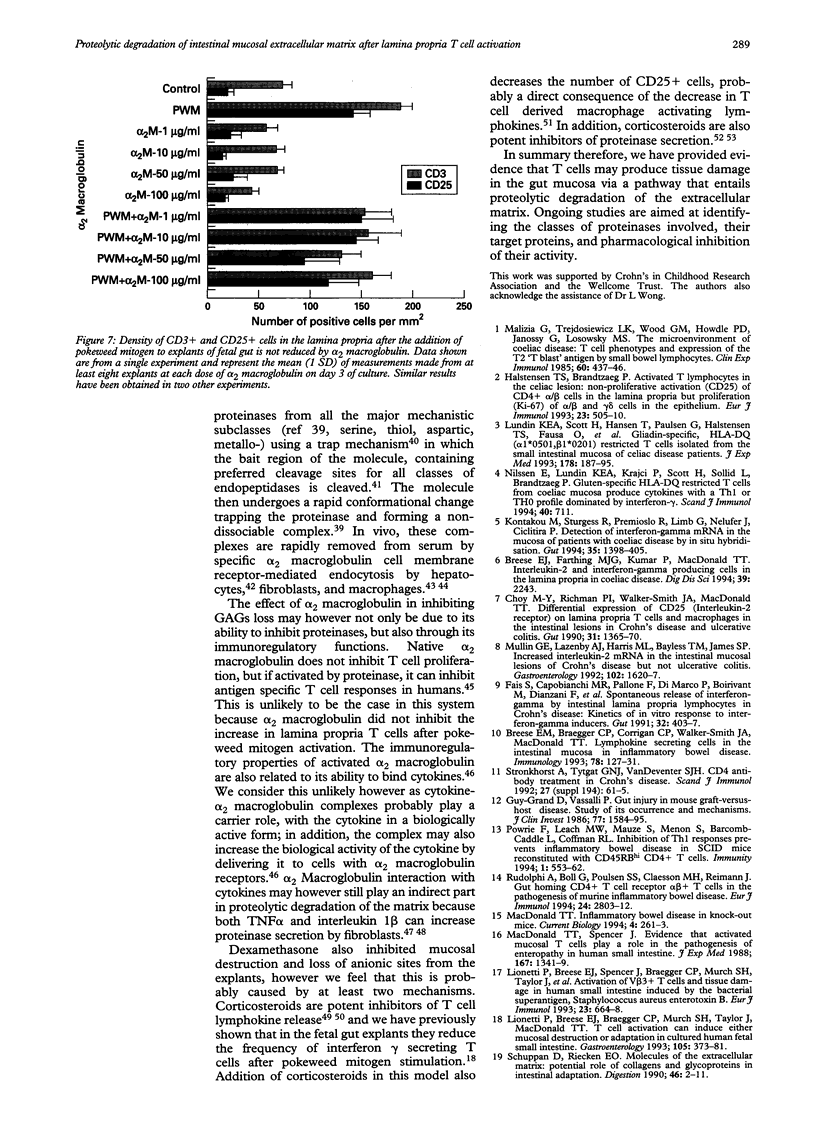
BACKGROUND: Proteoglycans, consisting of glycosaminoglycan (GAG) side chains covalently linked to a protein core, are a major component of the extracellular matrix of the intestinal lamina propria. AIMS: This study investigated the effects of lamina propria T cell activation on the proteoglycan component of the matrix. METHODS: The high degree of sulphation of GAGs means that they are polyanionic and thus can be visualised in tissue sections by means of colloidal-gold labelled cationic probes. RESULTS: In human fetal small intestine there is a dense meshwork of anionic residues in the lamina propria and basement membrane. When explants of human fetal small intestine are cultured ex vivo, and resident lamina propria T cells are activated with pokeweed mitogen, mucosal destruction occurs within three days. This is associated with the rapid loss of anionic sites from the lamina propria. Dermatan sulphate proteoglycan is lost from the tissue and is present at increased concentrations in the organ culture supernatants, indicating that T cell activation has led to solubilisation of lamina propria proteoglycans. Tissue destruction and loss of anionic residues are inhibited in a dose dependent fashion by dexamethasone, and by the protease inhibitor, alpha 2 macroglobulin. CONCLUSIONS: Proteolytic degradation of the lamina propria may therefore be a mechanism by which T cell hypersensitivity injures the intestinal mucosa.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Wong-Staal F., Gallo R. C. Dexamethasone-mediated inhibition of human T cell growth factor and gamma-interferon messenger RNA. J Immunol. 1984 Jul;133(1):273–276. [PubMed] [Google Scholar]
- Autrup H., Barrett L. A., Jackson F. E., Jesudason M. L., Stoner G., Phelps P., Trump B. F., Harris C. C. Explant culture of human colon. Gastroenterology. 1978 Jun;74(6):1248–1257. [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J. F., Vachon P. H., Chartrand S. Immunolocalization of extracellular matrix components during organogenesis in the human small intestine. Anat Embryol (Berl) 1991;183(4):363–369. doi: 10.1007/BF00196837. [DOI] [PubMed] [Google Scholar]
- Borth W. Alpha 2-macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 1992 Dec;6(15):3345–3353. doi: 10.1096/fasebj.6.15.1281457. [DOI] [PubMed] [Google Scholar]
- Breese E. J., Kumar P., Farthing M. J., MacDonald T. T. Interleukin-2 and interferon-gamma producing cells in the lamina propria in celiac disease. Dig Dis Sci. 1994 Oct;39(10):2243–2243. doi: 10.1007/BF02090378. [DOI] [PubMed] [Google Scholar]
- Breese E., Braegger C. P., Corrigan C. J., Walker-Smith J. A., MacDonald T. T. Interleukin-2- and interferon-gamma-secreting T cells in normal and diseased human intestinal mucosa. Immunology. 1993 Jan;78(1):127–131. [PMC free article] [PubMed] [Google Scholar]
- Choy M. Y., Walker-Smith J. A., Williams C. B., MacDonald T. T. Differential expression of CD25 (interleukin-2 receptor) on lamina propria T cells and macrophages in the intestinal lesions in Crohn's disease and ulcerative colitis. Gut. 1990 Dec;31(12):1365–1370. doi: 10.1136/gut.31.12.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. D., Kobayashi D. K., Welgus H. G. Regulation of the expression of tissue inhibitor of metalloproteinases and collagenase by retinoids and glucocorticoids in human fibroblasts. J Clin Invest. 1987 Nov;80(5):1280–1288. doi: 10.1172/JCI113203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fais S., Capobianchi M. R., Pallone F., Di Marco P., Boirivant M., Dianzani F., Torsoli A. Spontaneous release of interferon gamma by intestinal lamina propria lymphocytes in Crohn's disease. Kinetics of in vitro response to interferon gamma inducers. Gut. 1991 Apr;32(4):403–407. doi: 10.1136/gut.32.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S. R., Rosenberg M. R., Ney K. A., Michalopoulos G., Pizzo S. V. Binding of alpha 2-macroglobulin to hepatocytes: mechanism of in vivo clearance. Biochem Biophys Res Commun. 1985 Apr 30;128(2):795–802. doi: 10.1016/0006-291x(85)90117-2. [DOI] [PubMed] [Google Scholar]
- Garside P., Bunce C., Tomlinson R. C., Nichols B. L., Mowat A. M. Analysis of enteropathy induced by tumour necrosis factor alpha. Cytokine. 1993 Jan;5(1):24–30. doi: 10.1016/1043-4666(93)90020-6. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Vassalli P. Gut injury in mouse graft-versus-host reaction. Study of its occurrence and mechanisms. J Clin Invest. 1986 May;77(5):1584–1595. doi: 10.1172/JCI112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstensen T. S., Brandtzaeg P. Activated T lymphocytes in the celiac lesion: non-proliferative activation (CD25) of CD4+ alpha/beta cells in the lamina propria but proliferation (Ki-67) of alpha/beta and gamma/delta cells in the epithelium. Eur J Immunol. 1993 Feb;23(2):505–510. doi: 10.1002/eji.1830230231. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Nielsen M. L. Analysis of macrophage surface receptors. II. Internalization of alpha-macroglobulin . trypsin complexes by rabbit alveolar macrophages. J Biol Chem. 1979 Aug 10;254(15):7329–7335. [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Klein N. J., Shennan G. I., Heyderman R. S., Levin M. Alteration in glycosaminoglycan metabolism and surface charge on human umbilical vein endothelial cells induced by cytokines, endotoxin and neutrophils. J Cell Sci. 1992 Aug;102(Pt 4):821–832. doi: 10.1242/jcs.102.4.821. [DOI] [PubMed] [Google Scholar]
- Lander A. D. Targeting the glycosaminoglycan-binding sites on proteins. Chem Biol. 1994 Oct;1(2):73–78. doi: 10.1016/1074-5521(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Lionetti P., Breese E., Braegger C. P., Murch S. H., Taylor J., MacDonald T. T. T-cell activation can induce either mucosal destruction or adaptation in cultured human fetal small intestine. Gastroenterology. 1993 Aug;105(2):373–381. doi: 10.1016/0016-5085(93)90710-t. [DOI] [PubMed] [Google Scholar]
- Lionetti P., Spencer J., Breese E. J., Murch S. H., Taylor J., MacDonald T. T. Activation of mucosal V beta 3+ T cells and tissue damage in human small intestine by the bacterial superantigen, Staphylococcus aureus enterotoxin B. Eur J Immunol. 1993 Mar;23(3):664–668. doi: 10.1002/eji.1830230314. [DOI] [PubMed] [Google Scholar]
- Lundin K. E., Scott H., Hansen T., Paulsen G., Halstensen T. S., Fausa O., Thorsby E., Sollid L. M. Gliadin-specific, HLA-DQ(alpha 1*0501,beta 1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J Exp Med. 1993 Jul 1;178(1):187–196. doi: 10.1084/jem.178.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T. Gastrointestinal inflammation. Inflammatory bowel disease in knockout mice. Curr Biol. 1994 Mar 1;4(3):261–263. doi: 10.1016/s0960-9822(00)00060-9. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med. 1988 Apr 1;167(4):1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Spencer J. Ontogeny of the mucosal immune response. Springer Semin Immunopathol. 1990;12(2-3):129–137. doi: 10.1007/BF00197501. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Weinel A., Spencer J. HLA-DR expression in human fetal intestinal epithelium. Gut. 1988 Oct;29(10):1342–1348. doi: 10.1136/gut.29.10.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malizia G., Trejdosiewicz L. K., Wood G. M., Howdle P. D., Janossy G., Losowsky M. S. The microenvironment of coeliac disease: T cell phenotypes and expression of the T2 'T blast' antigen by small bowel lymphocytes. Clin Exp Immunol. 1985 May;60(2):437–446. [PMC free article] [PubMed] [Google Scholar]
- Mannhalter J. W., Borth W., Eibl M. M. Modulation of antigen-induced T cell proliferation by alpha 2M-trypsin complexes. J Immunol. 1986 Apr 15;136(8):2792–2799. [PubMed] [Google Scholar]
- McGuire-Goldring M. K., Murphy G., Gowen M., Meats J. E., Ebsworth N. M., Poll C., Reynolds J. J., Russell R. G. Effects of retinol and dexamethasone on cytokine-mediated control of metalloproteinases and their inhibitors by human articular chondrocytes and synovial cells in culture. Biochim Biophys Acta. 1983 Sep 22;763(2):129–139. doi: 10.1016/0167-4889(83)90036-8. [DOI] [PubMed] [Google Scholar]
- Meikle M. C., Atkinson S. J., Ward R. V., Murphy G., Reynolds J. J. Gingival fibroblasts degrade type I collagen films when stimulated with tumor necrosis factor and interleukin 1: evidence that breakdown is mediated by metalloproteinases. J Periodontal Res. 1989 May;24(3):207–213. doi: 10.1111/j.1600-0765.1989.tb02007.x. [DOI] [PubMed] [Google Scholar]
- Monk T., Spencer J., Cerf-Bensussan N., MacDonald T. T. Stimulation of mucosal T cells in situ with anti-CD3 antibody: location of the activated T cells and their distribution within the mucosal micro-environment. Clin Exp Immunol. 1988 Nov;74(2):216–222. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M. Antibodies to IFN-gamma prevent immunologically mediated intestinal damage in murine graft-versus-host reaction. Immunology. 1989 Sep;68(1):18–23. [PMC free article] [PubMed] [Google Scholar]
- Mullin G. E., Lazenby A. J., Harris M. L., Bayless T. M., James S. P. Increased interleukin-2 messenger RNA in the intestinal mucosal lesions of Crohn's disease but not ulcerative colitis. Gastroenterology. 1992 May;102(5):1620–1627. doi: 10.1016/0016-5085(92)91722-g. [DOI] [PubMed] [Google Scholar]
- Murch S. H., MacDonald T. T., Walker-Smith J. A., Levin M., Lionetti P., Klein N. J. Disruption of sulphated glycosaminoglycans in intestinal inflammation. Lancet. 1993 Mar 20;341(8847):711–714. doi: 10.1016/0140-6736(93)90485-y. [DOI] [PubMed] [Google Scholar]
- Powrie F., Leach M. W., Mauze S., Menon S., Caddle L. B., Coffman R. L. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994 Oct;1(7):553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Przemioslo R. T., Kontakou M., Nobili V., Ciclitira P. J. Raised pro-inflammatory cytokines interleukin 6 and tumour necrosis factor alpha in coeliac disease mucosa detected by immunohistochemistry. Gut. 1994 Oct;35(10):1398–1403. doi: 10.1136/gut.35.10.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przemioslo R. T., Lundin K. E., Sollid L. M., Nelufer J., Ciclitira P. J. Histological changes in small bowel mucosa induced by gliadin sensitive T lymphocytes can be blocked by anti-interferon gamma antibody. Gut. 1995 Jun;36(6):874–879. doi: 10.1136/gut.36.6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Abidi A. H., Alpers J. D., Hoover R. G., Robb R. J., Nowell P. C. Effect of cyclosporin A and dexamethasone on interleukin 2 receptor gene expression. J Immunol. 1986 Jul 1;137(1):150–154. [PubMed] [Google Scholar]
- Rudolphi A., Boll G., Poulsen S. S., Claesson M. H., Reimann J. Gut-homing CD4+ T cell receptor alpha beta+ T cells in the pathogenesis of murine inflammatory bowel disease. Eur J Immunol. 1994 Nov;24(11):2803–2812. doi: 10.1002/eji.1830241134. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989 Aug 15;264(23):13369–13372. [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Schuppan D., Riecken E. O. Molecules of the extracellular matrix: potential role of collagens and glycoproteins in intestinal adaptation. Digestion. 1990;46 (Suppl 2):2–11. doi: 10.1159/000200360. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L. Alpha-macroglobulins: structure, shape, and mechanism of proteinase complex formation. J Biol Chem. 1989 Jul 15;264(20):11539–11542. [PubMed] [Google Scholar]
- Sottrup-Jensen L., Birkedal-Hansen H. Human fibroblast collagenase-alpha-macroglobulin interactions. Localization of cleavage sites in the bait regions of five mammalian alpha-macroglobulins. J Biol Chem. 1989 Jan 5;264(1):393–401. [PubMed] [Google Scholar]
- Straight D. L., Jakoi L., McKee P. A., Snyderman R. Binding of alpha 2-macroglobulin-thrombin complexes and methylamine-treated alpha 2-macroglobulin to human blood monocytes. Biochemistry. 1988 Apr 19;27(8):2885–2890. doi: 10.1021/bi00408a033. [DOI] [PubMed] [Google Scholar]
- Thomas R., Schürmann G., Lionetti P., Pender S. L., MacDonald T. T. T cell receptor V beta expression in human intestine: regional variation in postnatal intestine and biased usage in fetal gut. Gut. 1996 Feb;38(2):190–195. doi: 10.1136/gut.38.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]