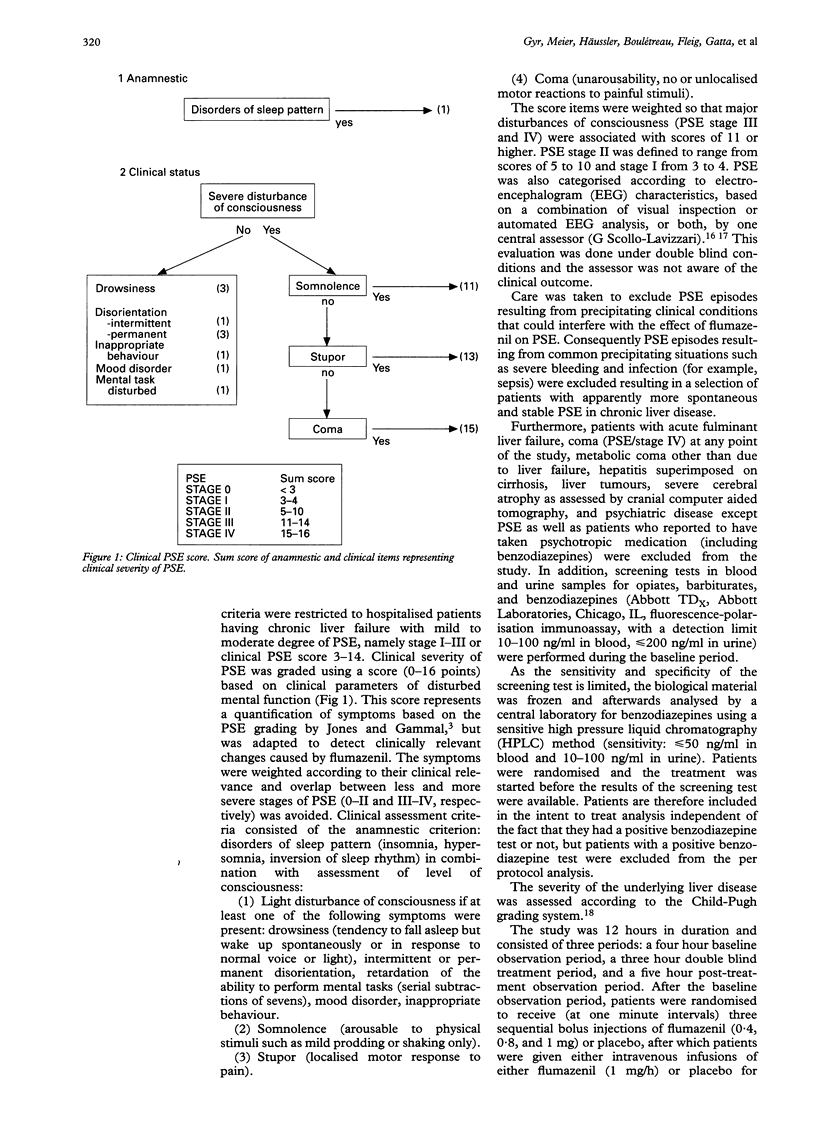
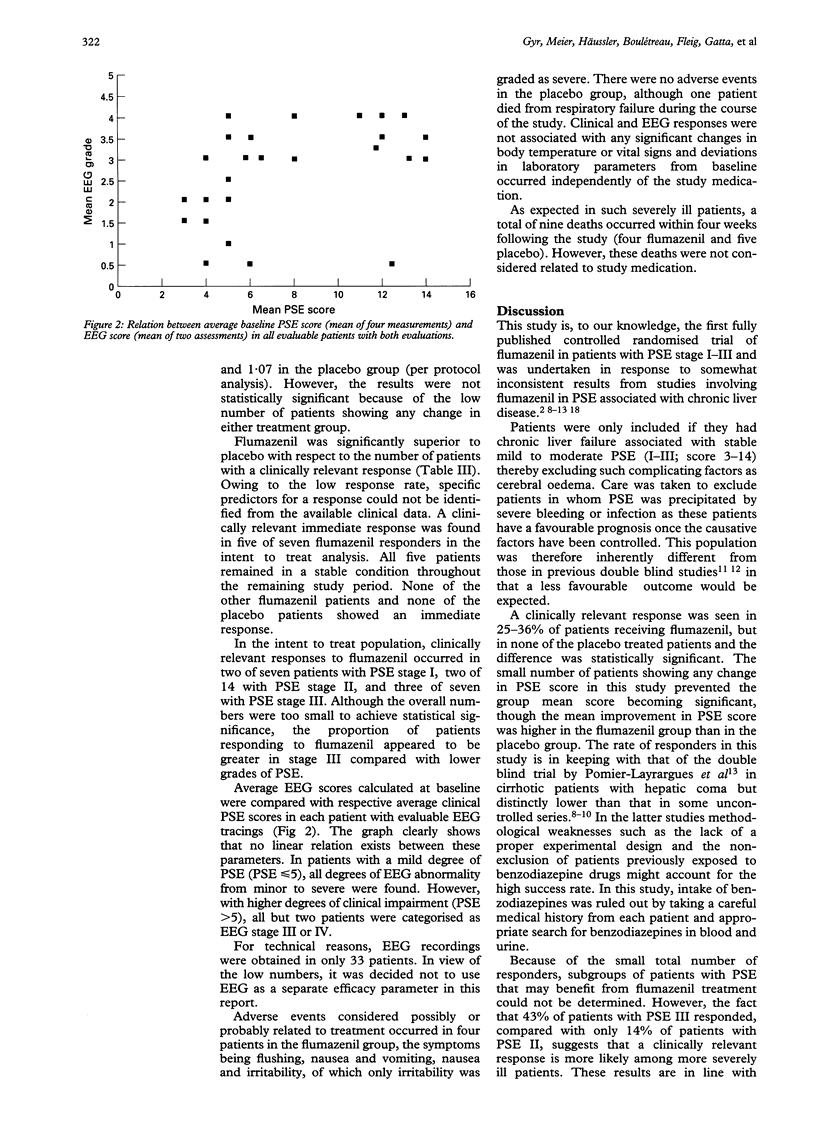
Abstract
BACKGROUND: Portal systemic encephalopathy (PSE) is a complex neuropsychiatric syndrome associated with hepatic failure. Small scale studies have shown the benzodiazepine receptor antagonist flumazenil to be effective in ameliorating PSE. AIMS: To determine the efficacy of flumazenil in patients with non-comatous mild to moderate PSE (stages I to III) due to severe chronic liver disease. PATIENTS: 49 male and female adults without symptoms of severe bleeding and sepsis and who screened negative for benzodiazepine in both blood and urine, were included in the study. METHODS: Patients were randomised to receive either three sequential bolus injections of flumazenil (0.4, 0.8, and 1 mg) or placebo at one minute intervals, followed by intravenous infusions of either flumazenil (1 mg/h) or placebo for three hours. Clinical PSE grading and vital signs were assessed hourly during baseline and post-treatment periods and half hourly during treatment. The main outcome measures were improvement in group average PSE score and reduction of two points in individual PSE score (clinically relevant improvement). RESULTS: The mean average improvement in the PSE score in the subjects treated with flumazenil was not statistically significantly different from placebo. However, for patients showing clinically relevant improvement, the difference between flumazenil and placebo was statistically significant (seven of 28 v none of 21; p = 0.015). Flumazenil was well tolerated. CONCLUSIONS: A subgroup of patients with PSE resulting from chronic liver disease may benefit from the administration of flumazenil.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrein R., Hetzel W., Hartmann D., Lorscheid T. Clinical pharmacology of flumazenil. Eur J Anaesthesiol Suppl. 1988;2:65–80. [PubMed] [Google Scholar]
- Bansky G., Meier P. J., Riederer E., Walser H., Ziegler W. H., Schmid M. Effects of the benzodiazepine receptor antagonist flumazenil in hepatic encephalopathy in humans. Gastroenterology. 1989 Sep;97(3):744–750. doi: 10.1016/0016-5085(89)90647-1. [DOI] [PubMed] [Google Scholar]
- Basile A. S., Harrison P. M., Hughes R. D., Gu Z. Q., Pannell L., McKinney A., Jones E. A., Williams R. Relationship between plasma benzodiazepine receptor ligand concentrations and severity of hepatic encephalopathy. Hepatology. 1994 Jan;19(1):112–121. [PubMed] [Google Scholar]
- Basile A. S., Jones E. A., Skolnick P. The pathogenesis and treatment of hepatic encephalopathy: evidence for the involvement of benzodiazepine receptor ligands. Pharmacol Rev. 1991 Mar;43(1):27–71. [PubMed] [Google Scholar]
- Bassett M. L., Mullen K. D., Skolnick P., Jones E. A. Amelioration of hepatic encephalopathy by pharmacologic antagonism of the GABAA-benzodiazepine receptor complex in a rabbit model of fulminant hepatic failure. Gastroenterology. 1987 Nov;93(5):1069–1077. doi: 10.1016/0016-5085(87)90571-3. [DOI] [PubMed] [Google Scholar]
- Cadranel J. F., el Younsi M., Pidoux B., Zylberberg P., Benhamou Y., Valla D., Opolon P. Flumazenil therapy for hepatic encephalopathy in cirrhotic patients: a double-blind pragmatic randomized, placebo study. Eur J Gastroenterol Hepatol. 1995 Apr;7(4):325–329. [PubMed] [Google Scholar]
- Grimm G., Ferenci P., Katzenschlager R., Madl C., Schneeweiss B., Laggner A. N., Lenz K., Gangl A. Improvement of hepatic encephalopathy treated with flumazenil. Lancet. 1988 Dec 17;2(8625):1392–1394. doi: 10.1016/s0140-6736(88)90587-9. [DOI] [PubMed] [Google Scholar]
- Haefely W. The preclinical pharmacology of flumazenil. Eur J Anaesthesiol Suppl. 1988;2:25–36. [PubMed] [Google Scholar]
- Kennedy J., Parbhoo S. P., MacGillivray B., Sherlock S. Effect of extracorporeal liver perfusion on the electroencephalogram of patients in coma due to acute liver failure. Q J Med. 1973 Jul;42(167):549–561. [PubMed] [Google Scholar]
- Meier R., Gyr K. Treatment of hepatic encephalopathy (HE) with the benzodiazepine antagonist flumazenil: a pilot study. Eur J Anaesthesiol Suppl. 1988;2:139–146. [PubMed] [Google Scholar]
- Mullen K. D., Szauter K. M., Kaminsky-Russ K. "Endogenous" benzodiazepine activity in body fluids of patients with hepatic encephalopathy. Lancet. 1990 Jul 14;336(8707):81–83. doi: 10.1016/0140-6736(90)91594-z. [DOI] [PubMed] [Google Scholar]
- Pomier-Layrargues G., Giguère J. F., Lavoie J., Perney P., Gagnon S., D'Amour M., Wells J., Butterworth R. F. Flumazenil in cirrhotic patients in hepatic coma: a randomized double-blind placebo-controlled crossover trial. Hepatology. 1994 Jan;19(1):32–37. [PubMed] [Google Scholar]
- Pugh R. N., Murray-Lyon I. M., Dawson J. L., Pietroni M. C., Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973 Aug;60(8):646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- Van der Rijt C. C., Schalm S. W., De Groot G. H., De Vlieger M. Objective measurement of hepatic encephalopathy by means of automated EEG analysis. Electroencephalogr Clin Neurophysiol. 1984 May;57(5):423–426. doi: 10.1016/0013-4694(84)90071-3. [DOI] [PubMed] [Google Scholar]


