Abstract
BACKGROUND: 6-Mercaptopurine (6-MP) has confirmed short and longterm efficacy in the treatment of IBD. However, the relation between its metabolism, efficacy, and side effects is not well understood. AIMS: To assay 6-MP metabolites and to correlate levels with drug compliance, disease activity, and adverse effects of treatment. PATIENTS: Heparinised blood was obtained prior to daily administration of 6-MP in 25 adolescent Crohn's disease patients (14 ileocolitis, 11 colitis) receiving 1.2 (range 0.4-1.6) mg/kg/day for a mean of 17 (range 4-65) months. METHODS: Erythrocyte free bases 6-thioguanine (6-TG) and 6-methyl-mercaptopurine (6-MMP) were measured (pmol/8 x 10(8) red blood cells) using reverse phase high performance liquid chromatography. RESULTS: Disease activity (modified Harvey-Bradshaw index) improved significantly with 6-MP (p = 0.001). Clinical remission was achieved in 72% of patients, who stopped taking prednisone, or were successfully weaned to a low alternate day dose (< 0.4 mg/kg/OD). Remission correlated well with erythrocyte 6-TG (p < 0.05), but not 6-MMP levels. Neutropenia was associated with 6-MP use (p < 0.005), but did not correlate with erythrocyte 6-MP metabolite levels. One patient refractory to 6-MP had 6-TG, but no measureable 6-MMP production, suggesting deficient thiopurine methyl-transferase activity or poor compliance. 6-MP induced complications (hepatitis, pancreatitis, and marrow suppression) were generally associated with increased 6-MMP levels. CONCLUSIONS: These results suggest that high performance liquid chromatography measurement of erythrocyte 6-MP metabolites may provide a quantitative assessment of patient responsiveness and compliance to treatment. The data support an immunosuppressive role for 6-TG, and potential cytotoxicity of raised 6-MMP levels.
Full text
PDF
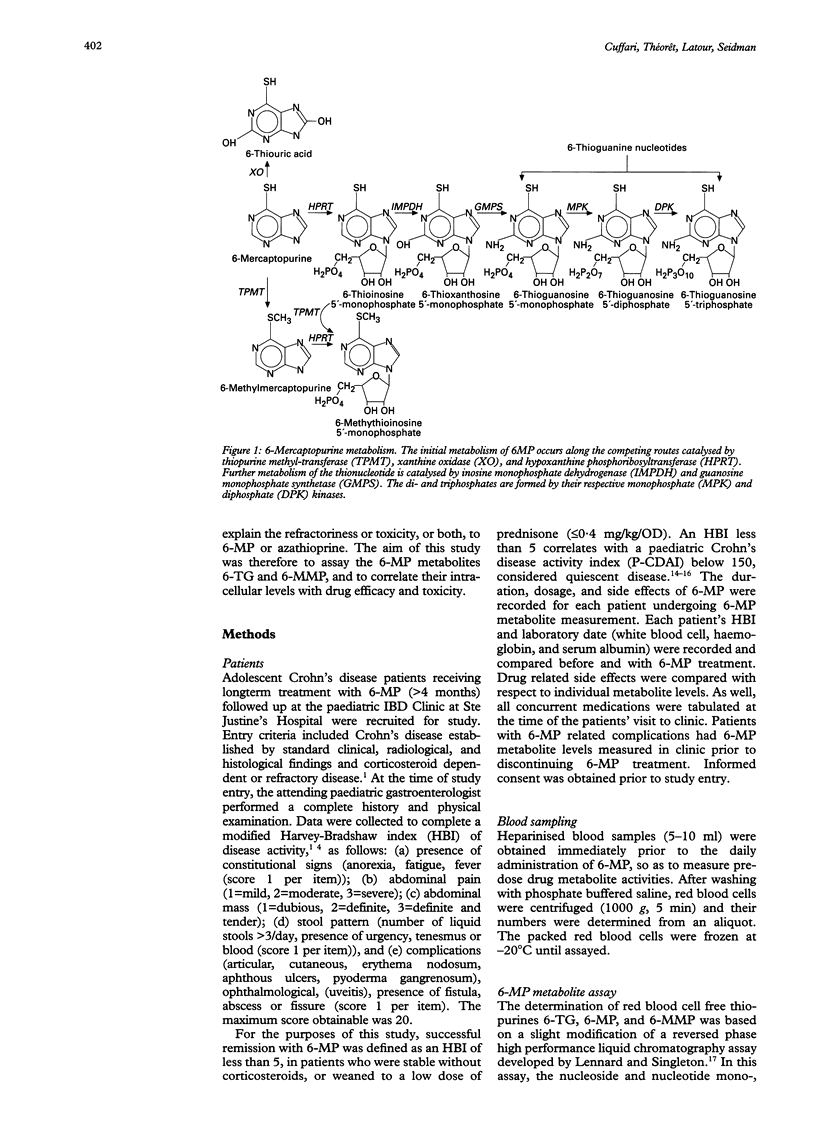
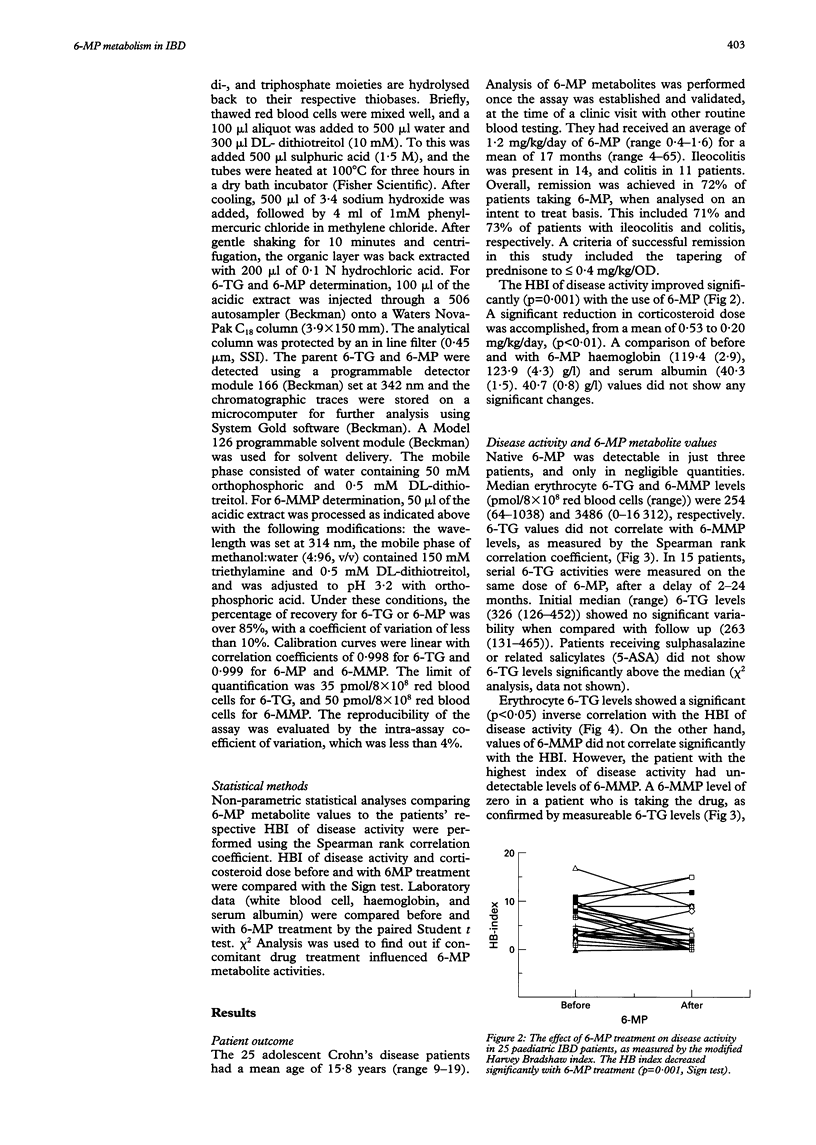
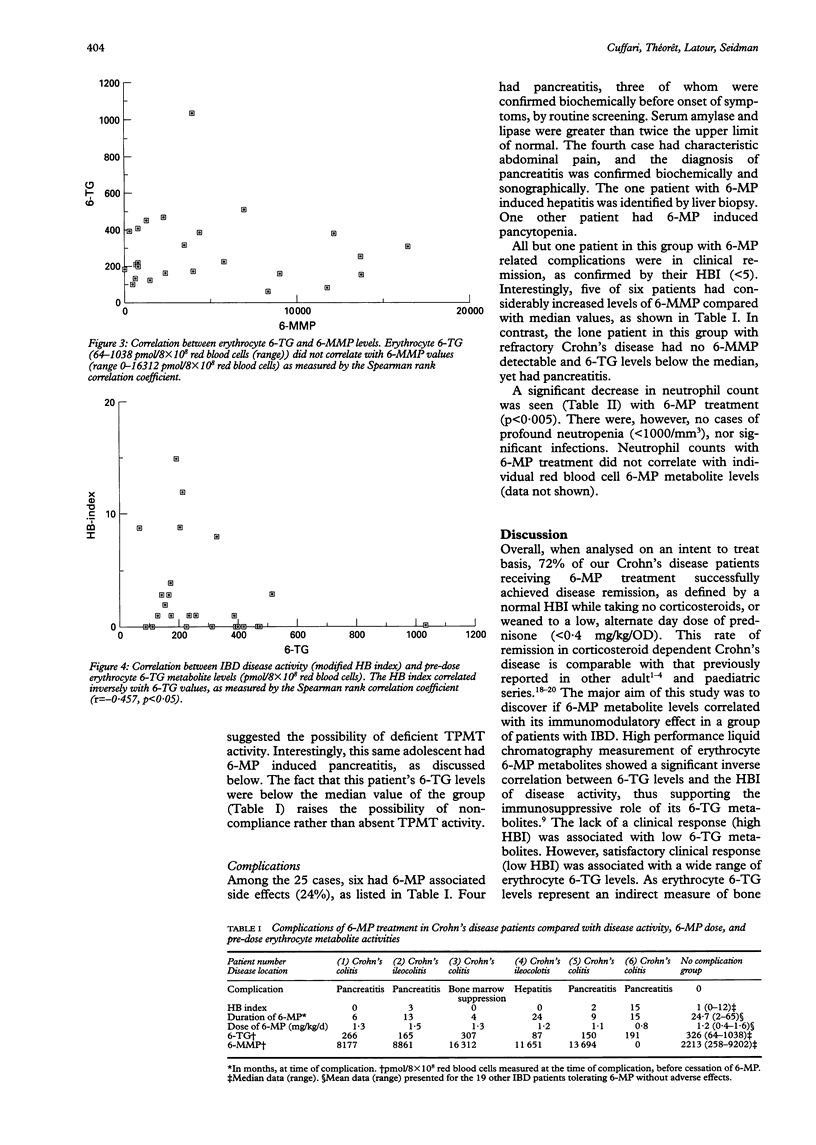


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belli D. C., Seidman E., Bouthillier L., Weber A. M., Roy C. C., Pletincx M., Beaulieu M., Morin C. L. Chronic intermittent elemental diet improves growth failure in children with Crohn's disease. Gastroenterology. 1988 Mar;94(3):603–610. doi: 10.1016/0016-5085(88)90230-2. [DOI] [PubMed] [Google Scholar]
- Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976 Mar;70(3):439–444. [PubMed] [Google Scholar]
- Bostrom B., Erdmann G. Cellular pharmacology of 6-mercaptopurine in acute lymphoblastic leukemia. Am J Pediatr Hematol Oncol. 1993 Feb;15(1):80–86. [PubMed] [Google Scholar]
- Brogan M., Hiserodt J., Oliver M., Stevens R., Korelitz B., Targan S. The effect of 6-mercaptopurine on natural killer-cell activities in Crohn's disease. J Clin Immunol. 1985 May;5(3):204–211. doi: 10.1007/BF00915512. [DOI] [PubMed] [Google Scholar]
- Bökkerink J. P., Stet E. H., De Abreu R. A., Damen F. J., Hulscher T. W., Bakker M. A., van Baal J. A. 6-Mercaptopurine: cytotoxicity and biochemical pharmacology in human malignant T-lymphoblasts. Biochem Pharmacol. 1993 Apr 6;45(7):1455–1463. doi: 10.1016/0006-2952(93)90045-x. [DOI] [PubMed] [Google Scholar]
- Colonna T., Korelitz B. I. The role of leukopenia in the 6-mercaptopurine-induced remission of refractory Crohn's disease. Am J Gastroenterol. 1994 Mar;89(3):362–366. [PubMed] [Google Scholar]
- Ewe K., Press A. G., Singe C. C., Stufler M., Ueberschaer B., Hommel G., Meyer zum Büschenfelde K. H. Azathioprine combined with prednisolone or monotherapy with prednisolone in active Crohn's disease. Gastroenterology. 1993 Aug;105(2):367–372. doi: 10.1016/0016-5085(93)90709-l. [DOI] [PubMed] [Google Scholar]
- Harvey R. F., Bradshaw J. M. A simple index of Crohn's-disease activity. Lancet. 1980 Mar 8;1(8167):514–514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- Korelitz B. I., Adler D. J., Mendelsohn R. A., Sacknoff A. L. Long-term experience with 6-mercaptopurine in the treatment of Crohn's disease. Am J Gastroenterol. 1993 Aug;88(8):1198–1205. [PubMed] [Google Scholar]
- Lennard L., Gibson B. E., Nicole T., Lilleyman J. S. Congenital thiopurine methyltransferase deficiency and 6-mercaptopurine toxicity during treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1993 Nov;69(5):577–579. doi: 10.1136/adc.69.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L., Lilleyman J. S. Variable mercaptopurine metabolism and treatment outcome in childhood lymphoblastic leukemia. J Clin Oncol. 1989 Dec;7(12):1816–1823. doi: 10.1200/JCO.1989.7.12.1816. [DOI] [PubMed] [Google Scholar]
- Lennard L., Rees C. A., Lilleyman J. S., Maddocks J. L. Childhood leukaemia: a relationship between intracellular 6-mercaptopurine metabolites and neutropenia. Br J Clin Pharmacol. 1983 Oct;16(4):359–363. doi: 10.1111/j.1365-2125.1983.tb02178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L., Singleton H. J. High-performance liquid chromatographic assay of human red blood cell thiopurine methyltransferase activity. J Chromatogr B Biomed Appl. 1994 Nov 4;661(1):25–33. doi: 10.1016/0378-4347(94)00327-0. [DOI] [PubMed] [Google Scholar]
- Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol. 1992;43(4):329–339. doi: 10.1007/BF02220605. [DOI] [PubMed] [Google Scholar]
- Lennard L., Van Loon J. A., Weinshilboum R. M. Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther. 1989 Aug;46(2):149–154. doi: 10.1038/clpt.1989.119. [DOI] [PubMed] [Google Scholar]
- Markowitz J., Daum F., Cohen S. A., Glassman M., Holmes R. D., Piccoli D., Rossi T. M., Treem W. R., Ulshen M. H., Winter H. S. Immunology of inflammatory bowel disease: summary of the proceedings of the Subcommittee on Immunosuppressive Use in IBD. J Pediatr Gastroenterol Nutr. 1991 May;12(4):411–423. [PubMed] [Google Scholar]
- Markowitz J., Grancher K., Mandel F., Daum F. Immunosuppressive therapy in pediatric inflammatory bowel disease: results of a survey of the North American Society for Pediatric Gastroenterology and Nutrition. Subcommittee on Immunosuppressive Use of the Pediatric IBD Collaborative Research Forum. Am J Gastroenterol. 1993 Jan;88(1):44–48. [PubMed] [Google Scholar]
- Markowitz J., Rosa J., Grancher K., Aiges H., Daum F. Long-term 6-mercaptopurine treatment in adolescents with Crohn's disease. Gastroenterology. 1990 Nov;99(5):1347–1351. doi: 10.1016/0016-5085(90)91160-8. [DOI] [PubMed] [Google Scholar]
- O'Brien J. J., Bayless T. M., Bayless J. A. Use of azathioprine or 6-mercaptopurine in the treatment of Crohn's disease. Gastroenterology. 1991 Jul;101(1):39–46. doi: 10.1016/0016-5085(91)90457-v. [DOI] [PubMed] [Google Scholar]
- Pearson D. C., May G. R., Fick G. H., Sutherland L. R. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995 Jul 15;123(2):132–142. doi: 10.7326/0003-4819-123-2-199507150-00009. [DOI] [PubMed] [Google Scholar]
- Perrault J., Greseth J. L., Tremaine W. J. 6-mercaptopurine therapy in selected cases of corticosteroid-dependent Crohn's disease. Mayo Clin Proc. 1991 May;66(5):480–484. doi: 10.1016/s0025-6196(12)62388-x. [DOI] [PubMed] [Google Scholar]
- Present D. H., Meltzer S. J., Krumholz M. P., Wolke A., Korelitz B. I. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med. 1989 Oct 15;111(8):641–649. doi: 10.7326/0003-4819-111-8-641. [DOI] [PubMed] [Google Scholar]
- Stet E. H., De Abreu R. A., Bökkerink J. P., Vogels-Mentink T. M., Lambooy L. H., Trijbels F. J., Trueworthy R. C. Reversal of 6-mercaptopurine and 6-methylmercaptopurine ribonucleoside cytotoxicity by amidoimidazole carboxamide ribonucleoside in Molt F4 human malignant T-lymphoblasts. Biochem Pharmacol. 1993 Aug 3;46(3):547–550. doi: 10.1016/0006-2952(93)90534-4. [DOI] [PubMed] [Google Scholar]
- Szumlanski C. L., Weinshilboum R. M. Sulphasalazine inhibition of thiopurine methyltransferase: possible mechanism for interaction with 6-mercaptopurine and azathioprine. Br J Clin Pharmacol. 1995 Apr;39(4):456–459. doi: 10.1111/j.1365-2125.1995.tb04478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhave M., Winter H. S., Grand R. J. Azathioprine in the treatment of children with inflammatory bowel disease. J Pediatr. 1990 Nov;117(5):809–814. doi: 10.1016/s0022-3476(05)83349-9. [DOI] [PubMed] [Google Scholar]
- Zimm S., Collins J. M., Riccardi R., O'Neill D., Narang P. K., Chabner B., Poplack D. G. Variable bioavailability of oral mercaptopurine. Is maintenance chemotherapy in acute lymphoblastic leukemia being optimally delivered? N Engl J Med. 1983 Apr 28;308(17):1005–1009. doi: 10.1056/NEJM198304283081705. [DOI] [PubMed] [Google Scholar]


