Abstract
BACKGROUND/AIMS: The role of host factors has been neglected in studies of the pathogenesis of Helicobacter associated disease. The aim of this study was to assess the response of different mouse strains to infection with a single strain of Helicobacter felis. METHOD: Six strains of inbred mice were infected with the identical H felis culture and were killed at one month, two months, and six months after infection to assess histopathological changes. In addition, two strains of mice were infected with a mouse adapted strain of H pylori and examined at six months after infection. RESULTS: In SJL, C3H/He, DBA/2, and C57BL/6 infected mice, severe to moderate chronic active gastritis was observed only in the body of the stomach, which increased in severity over time with specialised cells in the body glands being replaced. As the severity of this damage in the body increased and atrophic changes were seen, the level of bacterial colonisation of the antrum decreased. In contrast, in BALB/c and CBA mice, there was only mild gastritis in the antrum, no remarkable changes were detected in their body mucosa, and no atrophy was seen over time. In both these strains of mice, heavy bacterial colonisation was seen, which tended to increase over the period of the experiment. Of particular importance in this experiment was that bacterial colonisation was mainly restricted to the antrum yet the atrophy, when present, was only observed in the body of the stomach. H pylori infected C3H/He mice showed moderate colonisation of the antrum, which persisted up to six months with little development of atrophy. In contrast, H pylori in C57BL/6 mice showed excellent colonisation of the antrum at two months but six months after infection there was moderate to severe body atrophy, which was associated with a loss of bacteria from the antrum. CONCLUSIONS: These findings challenge current concepts of the development of Helicobacter induced atrophy in that active chronic gastritis of antrum or the body mucosa, or both, is not a prerequisite. They also suggest an autoimmune basis for the pathology although no autoantibody or antibody to the H+/K+ ATPase was detected. Loss of infecting helicobacters from the stomach together with development of an atrophic gastritis in the body of the stomach is similar to the pattern found in certain H pylori infected human subjects.
Full text
PDF



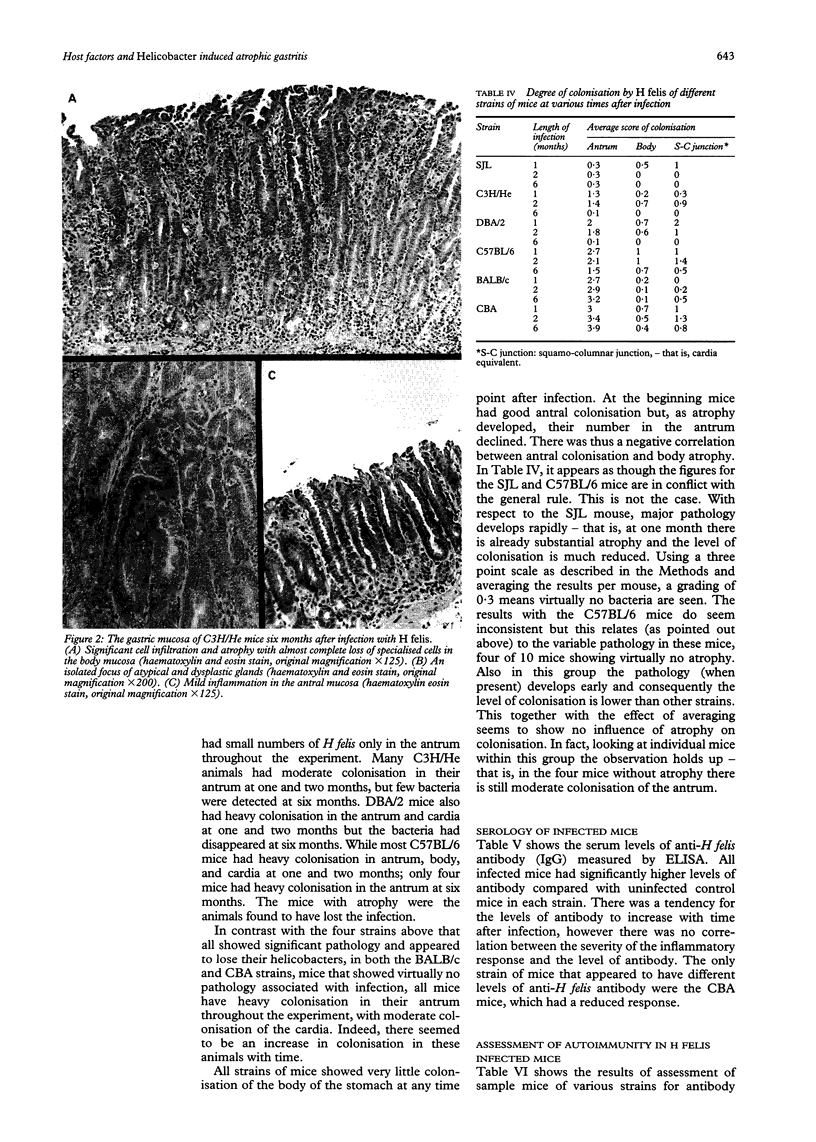

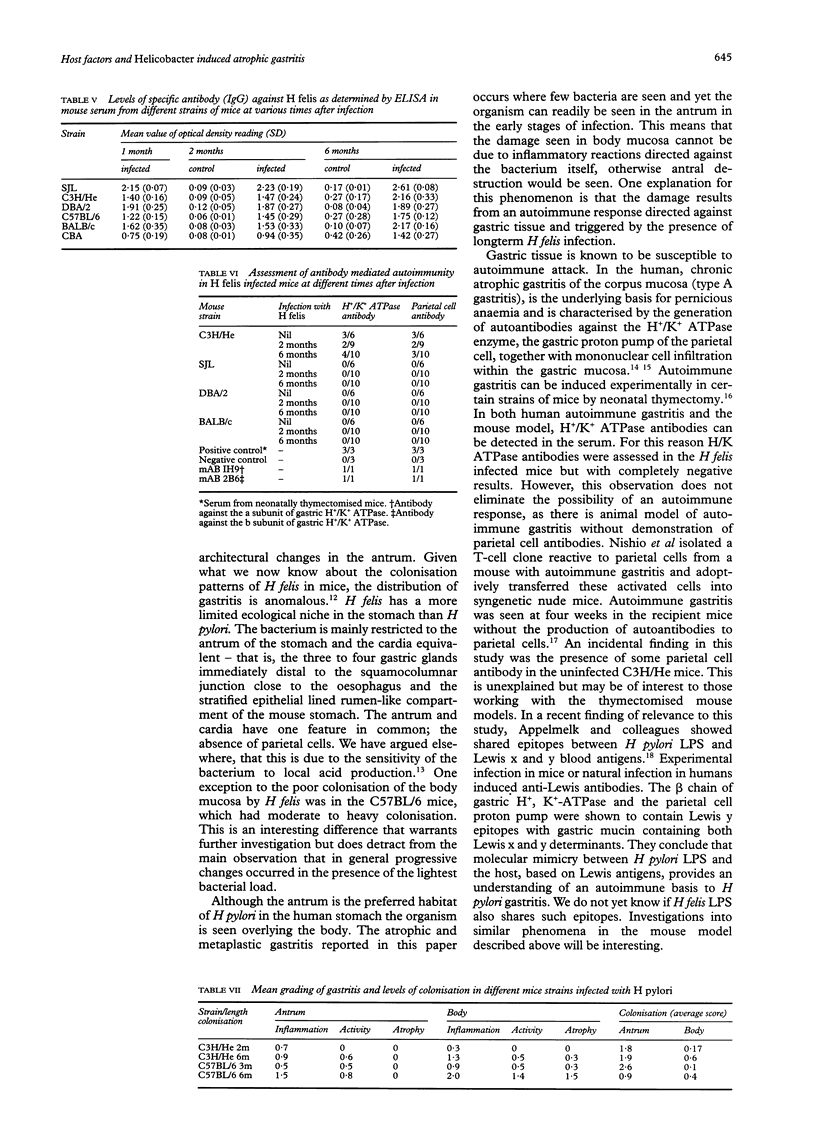



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderuccio F., Toh B. H., Tan S. S., Gleeson P. A., van Driel I. R. An autoimmune disease with multiple molecular targets abrogated by the transgenic expression of a single autoantigen in the thymus. J Exp Med. 1993 Aug 1;178(2):419–426. doi: 10.1084/jem.178.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelmelk B. J., Simoons-Smit I., Negrini R., Moran A. P., Aspinall G. O., Forte J. G., De Vries T., Quan H., Verboom T., Maaskant J. J. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 1996 Jun;64(6):2031–2040. doi: 10.1128/iai.64.6.2031-2040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang J. S., Callaghan J. M., Gleeson P. A., Toh B. H. Diagnostic ELISA for parietal cell autoantibody using tomato lectin-purified gastric H+/K(+)-ATPase (proton pump). Autoimmunity. 1992;12(1):1–7. doi: 10.3109/08916939209146123. [DOI] [PubMed] [Google Scholar]
- Correa P., Miller M. J. Helicobacter pylori and gastric atrophy--cancer paradoxes. J Natl Cancer Inst. 1995 Dec 6;87(23):1731–1732. doi: 10.1093/jnci/87.23.1731. [DOI] [PubMed] [Google Scholar]
- Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z., Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Covacci A., Farmery S. M., Xiang Z., Tompkins D. S., Perry S., Lindley I. J., Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995 Jan;48(1):41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Farmery S. M. Helicobacter pylori and gastric mucosal cytokines: evidence that CagA-positive strains are more virulent. Lab Invest. 1995 Dec;73(6):742–745. [PubMed] [Google Scholar]
- Danon S. J., O'Rourke J. L., Moss N. D., Lee A. The importance of local acid production in the distribution of Helicobacter felis in the mouse stomach. Gastroenterology. 1995 May;108(5):1386–1395. doi: 10.1016/0016-5085(95)90686-x. [DOI] [PubMed] [Google Scholar]
- Enno A., O'Rourke J. L., Howlett C. R., Jack A., Dixon M. F., Lee A. MALToma-like lesions in the murine gastric mucosa after long-term infection with Helicobacter felis. A mouse model of Helicobacter pylori-induced gastric lymphoma. Am J Pathol. 1995 Jul;147(1):217–222. [PMC free article] [PubMed] [Google Scholar]
- Faisal M. A., Russell R. M., Samloff I. M., Holt P. R. Helicobacter pylori infection and atrophic gastritis in the elderly. Gastroenterology. 1990 Nov;99(5):1543–1544. doi: 10.1016/0016-5085(90)91213-p. [DOI] [PubMed] [Google Scholar]
- Karlsson F. A., Burman P., Löf L., Mårdh S. Major parietal cell antigen in autoimmune gastritis with pernicious anemia is the acid-producing H+,K+-adenosine triphosphatase of the stomach. J Clin Invest. 1988 Feb;81(2):475–479. doi: 10.1172/JCI113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnes W. E., Jr, Samloff I. M., Siurala M., Kekki M., Sipponen P., Kim S. W., Walsh J. H. Positive serum antibody and negative tissue staining for Helicobacter pylori in subjects with atrophic body gastritis. Gastroenterology. 1991 Jul;101(1):167–174. doi: 10.1016/0016-5085(91)90474-y. [DOI] [PubMed] [Google Scholar]
- Kojima A., Prehn R. T. Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics. 1981;14(1-2):15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- Lee A., Chen M., Coltro N., O'Rourke J., Hazell S., Hu P., Li Y. Long term infection of the gastric mucosa with Helicobacter species does induce atrophic gastritis in an animal model of Helicobacter pylori infection. Zentralbl Bakteriol. 1993 Sep;280(1-2):38–50. doi: 10.1016/s0934-8840(11)80939-4. [DOI] [PubMed] [Google Scholar]
- Lee A., Dixon M. F., Danon S. J., Kuipers E., Mégraud F., Larsson H., Mellgård B. Local acid production and Helicobacter pylori: a unifying hypothesis of gastroduodenal disease. Eur J Gastroenterol Hepatol. 1995 May;7(5):461–465. [PubMed] [Google Scholar]
- Mohammadi M., Redline R., Nedrud J., Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect Immun. 1996 Jan;64(1):238–245. doi: 10.1128/iai.64.1.238-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini R., Lisato L., Zanella I., Cavazzini L., Gullini S., Villanacci V., Poiesi C., Albertini A., Ghielmi S. Helicobacter pylori infection induces antibodies cross-reacting with human gastric mucosa. Gastroenterology. 1991 Aug;101(2):437–445. doi: 10.1016/0016-5085(91)90023-e. [DOI] [PubMed] [Google Scholar]
- Nishio A., Hosono M., Watanabe Y., Sakai M., Okuma M., Masuda T. A conserved epitope on H+,K(+)-adenosine triphosphatase of parietal cells discerned by a murine gastritogenic T-cell clone. Gastroenterology. 1994 Nov;107(5):1408–1414. doi: 10.1016/0016-5085(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Siurala M., Sipponen P., Kekki M. Campylobacter pylori in a sample of Finnish population: relations to morphology and functions of the gastric mucosa. Gut. 1988 Jul;29(7):909–915. doi: 10.1136/gut.29.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland R. G., Baur S., Ashworth L. A., Taylor K. B. A correlative study of immunological phenomena in pernicious anaemia. Clin Exp Immunol. 1971 Jan;8(1):25–36. [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Cover T. L., Blaser M. J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993 May;61(5):1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]







