Abstract
VCAM-1 is one of the main ligands of VLA-4, an integrin that is highly expressed on the surface of mature B cells. Here we find that coexpression of VCAM-1 on an antigen-bearing membrane facilitates B-cell activation. Firstly, this is achieved by mediating B-cell tethering, which in turn increases the likelihood of a B cell to be activated. Secondly, VLA-4 synergizes with the B-cell receptor (BCR), providing B cells with tight adhesion and enhanced signalling. This dual role of VCAM-1 in promoting B-cell activation is predominantly effective when the affinity of the BCR for the antigen is low. In addition, we show that the VCAM-1 ectodomain alone is sufficient to carry out this function. However, it requires the transmembrane domain to segregate properly into a docking structure characteristic of the B-cell immunological synapse (IS). These results show that the VLA-4/VCAM-1 interaction during membrane antigen recognition enhances B-cell activation and this function appears to be independent of its final peripheral localization at the IS.
Keywords: affinity, antigen, B cell, BCR, synapse
Introduction
The activation of B lymphocytes is triggered by the specific binding of antigen to B-cell receptor (BCR). While the degree of activation is determined by the affinity of this interaction (Lanzavecchia, 1985; Batista and Neuberger, 1998; Kouskoff et al, 1998), B-cell activation is also regulated by the way in which antigen is presented (Goodnow et al, 1988; Lang et al, 1996). B cells respond well to soluble antigens; however, antigens tethered on a cell surface are especially potent in driving B-cell activation (Batista et al, 2001). This difference in effectiveness between soluble and membrane-bound antigen is particularly important in vivo where antigens are often encountered in the form of immune complexes (ICs) tethered on a cell surface by complement or Fc receptors (Szakal et al, 1988; Haberman and Shlomchik, 2003; Kosco-Vilbois, 2003).
We have recently shown that the interaction of leukocyte function-associated molecule-1 (LFA-1)/intercellular adhesion molecule-1 (ICAM-1) facilitates membrane antigen recognition by B cells (Carrasco et al, 2004). B-cell recognition of membrane-bound antigens leads to the formation of a mature immunological synapse (IS) where the BCR gathers antigen into a central cluster, surrounded by a peripheral ring of LFA-1 and ICAM-1 (Batista et al, 2001; Carrasco et al, 2004). This type of receptor distribution into defined supramolecular activation clusters (SMACs) was originally observed in T cells (Monks et al, 1998; Grakoui et al, 1999; Krummel et al, 2000). While considerable effort has been made to dissect the role and compartmentalization of LFA-1/ICAM-1 in this structure, very little is known about the function and localization of other integrins, such as very late antigen-4 (VLA-4).
VLA-4 (CD49d/CD29; α4β1) belongs to the integrin family of cell adhesion molecules and is expressed on all leucocytes, including B cells (Springer, 1990). VLA-4 mediates leucocyte tethering, rolling and firm adhesion by interacting with vascular adhesion molecule-1 (VCAM-1) on the endothelium (Alon et al, 1995; Berlin et al, 1995). Such functional flexibility is achieved by adopting different states of activation (Chen et al, 1999). Particularly in B cells, VLA-4 has been shown to be involved in the compartmentalization of B cells into peripheral lymphoid tissue (Lu and Cyster, 2002; Lo et al, 2003). It has also been implicated in promoting the physical interaction of B cells and follicular dendritic cells (FDCs) (Koopman et al, 1991). In addition, increased adhesion through this integrin has been observed in response to chemokines and BCR crosslinking (McLeod et al, 2002; Spaargaren et al, 2003).
VLA-4 ligand, VCAM-1, is constitutively expressed on FDCs (Freedman et al, 1990; Koopman et al, 1991), in high endothelial venules of peripheral and mesenteric lymph nodes (Berlin et al, 1995) and on bone marrow stromal cells (Miyake et al, 1991). Its expression can also be induced in endothelium by various inflammatory cytokines (IFNγ, TNFα) (Osborn et al, 1989). The ability to facilitate leucocyte adhesion to endothelium implies that VCAM-1 is an important factor in the initiation of an inflammatory response. Indeed, conditional VCAM-1 mutant mice present an impaired humoral immune response against T-cell-dependent antigens (Leuker et al, 2001). Moreover, the upregulation of VCAM-1 expression on endothelium is associated with several chronic inflammatory diseases (van Dinther-Janssen et al, 1991; Belmont et al, 1994). However, the function of VLA-4/VCAM-1 in B-cell recognition of membrane antigen has not been investigated.
In this study, we show that the interaction of VLA-4 with VCAM-1 can synergize with the BCR to promote adhesion of B cells to antigen-bearing membranes and thereby facilitate antigen recognition. We also show that VCAM-1 can cooperate with ICAM-1 to supply firm B-cell adhesion upon BCR activation. Remarkably, under shear stress conditions, the VLA-4/VCAM-1 interaction becomes a prerequisite for B-cell membrane-bound antigen recognition. This function is particularly striking when the affinity of the BCR for the antigen is low. Using target cells expressing VCAM-1 fused to green fluorescence protein (GFP), we show that this molecule participates in the formation of a docking structure that surrounds the BCR in the IS. However, VCAM-1 mutants lacking the transmembrane domain fail to be recruited to this docking structure or to be excluded from the central SMAC (cSMAC). These results point to a potential function for VLA-4/VCAM-1 in the recruitment and activation of B cells during an adaptive immune response.
Results
The experimental system employed in this study is based on the use of wild–type (WT) B cells, and B cells obtained from two BCR transgenic models, 3-83 and MD4 (Goodnow et al, 1988; Russell et al, 1991), which allows us to compare our observations. The 3-83 transgenic BCR binds with high affinity to the H-2Kk molecule, and also recognizes with substantially decreased affinity the peptides p31 and p11, which were derived from an M13 phage-display library (Table I; Kouskoff et al, 1998; Carrasco et al, 2004). B cells from MD4 transgenic mice carry an IgM+IgD+ BCR, which binds hen egg lysozyme (HEL) with an affinity of 5 × 1010 M−1 and mutated HELs with more than 10 000-fold decreased affinities (Table I; Batista and Neuberger, 1998; Carrasco et al, 2004).
Table 1.
Antigen and integrin ligand affinities
| Antigen | KA (× 106 M−1) | Integrin ligand | KA (106 M−1) |
|---|---|---|---|
| 3-83 binding | VCAM-1 | ||
| p31 | 65 | High-affinity VLA-4 | 10 |
| p11 | 7 | Low-affinity VLA-4 | ∼0.1 |
| p0 | — | ||
| HyHEL10 binding | ICAM-1 | ||
| HELWT | 50 000 | High-affinity LFA-1 | ∼2 |
| HELRD | 2080 | Low-affinity LFA-1 | ∼0.01 |
| HELKD | 30 | ||
| HELRKD | ∼3 | ||
| The KA values for the different peptides/3-83 antibody and HEL mutants/HyHEL10 antibody were obtained using the Biacore, as previously described (Batista and Neuberger, 1998; Carrasco et al, 2004). The values derived represent the affinity of the dimeric antibody for the peptide in the case of 3-83. The values of HyHEL10 for HELWT and the different HEL mutants account for the monomeric interaction. The KA values for the integrins VLA-4 (Chigaev et al, 2003) and LFA-1 (Lollo et al, 1993) in high- and low-affinity states were previously reported. | |||
The interaction of VLA-4 with VCAM-1 promotes B-cell adhesion to antigen-bearing membranes
To study the role of VCAM-1 during membrane antigen recognition, we generated a glycosylphosphatidylinositol-anchored version of VCAM-1 (GPI-VCAM-1) and asked whether its interaction with VLA-4 enhanced the capacity of B cells to form antigen-dependent and -independent contacts with planar lipid bilayers. As a positive control, naive B cells were tested for adhesion to bilayers that contained an equivalent density of ICAM-1 (50 molec/μm2). Quantification of the number of naive B cells attached to the membrane was carried out by interference reflection microscopy (IRM), which allows visualization of tight contact as dark areas (Figure 1A). At low densities of p31 (Table I), tight contacts were not detected unless VCAM-1 or ICAM-1 was present in the lipid bilayers (Figure 1A). The presence of VCAM-1 was able to support the binding of a larger number of B cells than ICAM-1 (Figure 1B). However, the area of contact of the B cells on ICAM-1 was larger than on VCAM-1 (Figure 1C). High concentration of antigen supported B-cell adhesion, and the presence of VCAM-1 or ICAM-1 did not significantly affect the level of adhesion. These results showed that tight adhesion requires membranes containing p31 at a density higher than 25 molec/μm2. In contrast, in the presence of VCAM-1 or ICAM-1, a reduced antigen density supported a similar level of adhesion (Figure 1B). Thus, similar to ICAM-1/LFA-1 (Carrasco et al, 2004), VCAM-1/VLA-4 facilitates adhesion under limited concentration of antigen and this function is regulated by BCR engagement of antigen.
Figure 1.
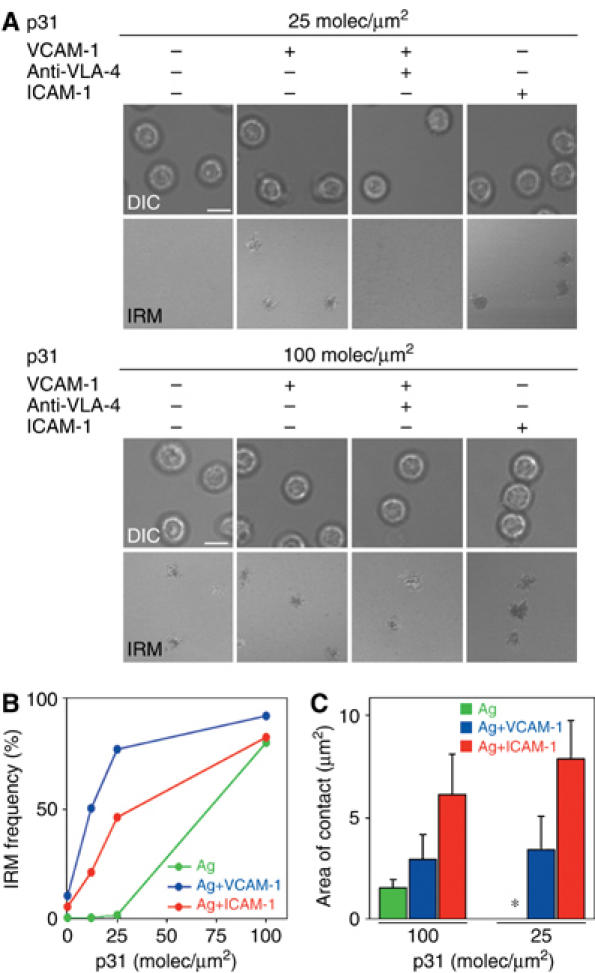
VLA-4/VCAM-1 interaction increases the adhesion of B cells to target membranes. (A) Differential interference contrast (DIC) and IRM images of 3-83 naive B cells settled onto planar lipid bilayers containing p31 antigen (100 molec/μm2, top panels; 25 molec/μm2, bottom panels) in the presence or absence of ICAM-1 (50 molec/μm2), VCAM-1 (50 molec/μm2) and/or neutralizing anti-mouse α4 mAbs. Scale bar, 5 μm. (B) Naive 3-83 B cells were evaluated for their capacity to form tight contacts on membranes loaded with p31 in the presence or absence of ICAM-1 or VCAM-1 at 50 molec/μm2. After 5–10 min, images were collected at each of the specified antigen densities and the number of cells showing tight contacts was determined. Data are representative of four different experiments. (C) Quantification of the area of B-cell contact with membranes containing p31 antigen at the indicated densities in the presence or absence of ICAM-1 or VCAM-1 at 50 molec/μm2. Data represent the mean of 40 cells in each case.
VLA-4 and VCAM-1 are involved during the first stages of the interaction
The area of contact between B cells and antigen-loaded membranes in the presence of ICAM-1 was around 6–7 μm2, whereas B cells bind to VCAM-1-containing membranes with areas of just 3 μm2 (Figure 1C). To test whether these two integrin pairs have a different function, we compared the adhesion of naive splenic B cells to VCAM-1, ICAM-1 and antigen-containing membranes under shear stress. Glass slides containing planar lipid bilayers were connected to a syringe pump to establish a laminar flow, and B cells were injected. While the nature of flow through the lymphatic tissues is still under investigation, it is known to be lower than that observed in postcapillary venules (1–10 dyn/cm2) (Atherton and Born, 1972). For this reason, we chose a low starting flow of 0.4 dyn/cm2, which was gradually decreased to 0.04 dyn/cm2. This allowed us to analyse the efficiency of B-cell binding to the membranes. We then slowly increased the shear stress to values up to 2.5 dyn/cm2, which might not fully represent the physiological conditions in the lymphatic tissue, but allowed us to evaluate the strength of adhesion of the captured cells.
As shown in Figure 2 and Supplementary Movie S1, 20% of the naive splenic 3-83-injected B cells were able to either tether or firmly attach onto membranes loaded with VCAM-1 during the injection period. Phenotypic characterization of the bound cells revealed that marginal zone B cells were able to bind slightly better to VCAM-1 than follicular B cells under the conditions of our assay (Supplementary Figure 1A). The tethering of B cells under shear stress was dependent on the density of VCAM-1, with efficient attachment at densities greater than 50 molec/μm2. Under these conditions, only 2% of the flowing B cells were able to roll on VCAM-1-bearing membranes and generally detached afterwards (Supplementary Figure 1B). However, as the flow rate was increased, tethered B cells were rapidly washed away, indicating that the interaction with VCAM-1 in the membrane was not very tight. In contrast, naive B cells did not adhere to artificial bilayers containing either ICAM-1 or antigen alone under shear stress, even at high densities of 100 molec/μm2, although such density supports static binding of naive B cells (see above). These results indicate that VCAM-1 was more efficient at capturing naive B cells than ICAM-1 or antigen under shear stress, suggesting that it is involved during the first stages of the interaction.
Figure 2.
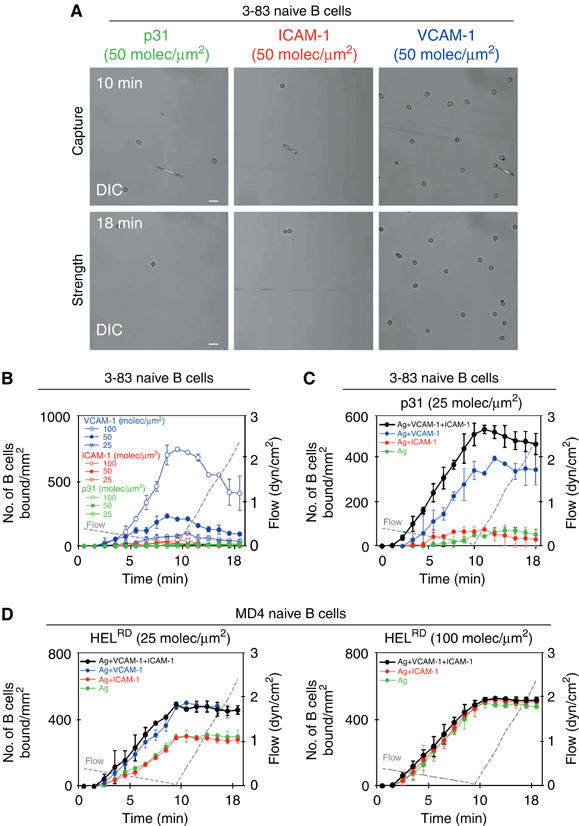
VLA-4/VCAM-1 interaction facilitates the capture of naive B cells and membrane-bound antigen recognition under shear stress. (A, B) 3-83 naive B cells were perfused at decreasing shear stress for 10 min (capture step), followed by injection of buffer at increasing shear stress for 8 min (strength step). DIC images at two different time points of the same representative field of p31, ICAM-1 and VCAM-1 membranes are shown in (A). The capacity to capture B cells and the strength of binding of lipid bilayers containing VCAM-1, ICAM-1 or p31 antigen (KA=6.5 × 107 M−1) at different densities (B), or of lipid bilayers containing p31 antigen in the presence or absence of VCAM-1 (50 molec/μm2) and/or ICAM-1 (50 molec/μm2) (C), were evaluated by counting B cells bound/mm2 at the indicated time points. (D) Lipid bilayers containing HELRD (KA=2 × 109 M−1) at the indicated densities in the presence or absence of VCAM-1 (50 molec/μm2) and/or ICAM-1 (50 molec/μm2) were evaluated for their capacity to capture MD4 naive B cells and the strength of binding as previously described. The data in (A–D) are representative of at least three experiments. Scale bar, 20 μm.
We then analysed the adhesion of B cells to membranes containing different combinations of VCAM-1, ICAM-1 and antigen. As shown in Figure 2C, inclusion of VCAM-1 on bilayers containing limited amounts of antigen enhanced B-cell tethering and firm adhesion when compared with B cells interacting with membranes containing antigen alone. An additional increase in the number of cells attached to the bilayers was observed when naive B cells were perfused over membranes containing a combination of VCAM-1, ICAM-1 and antigen. However, no rolling leading to firm adhesion could be detected. This absence of rolling may be due to the low shear stress conditions in which the assays were performed. Leucocyte rolling was observed in vivo within a range of 1.5–4 dyn/cm2 shear stress (Atherton and Born, 1972). B cells that had been activated by BCR crosslinking before perfusion on the membrane showed a greatly enhanced efficiency of adhesion to VCAM-1 and ICAM-1 (Supplementary Figure 1C). However, no enhanced attachment to the two integrin ligands was detected in the absence of BCR activation (Supplementary Figure 1D). From these data, we can conclude that VLA-4 can synergize with the BCR to promote B-cell capture and tight adhesion. Our experiments also show the inability of LFA-1 to initiate a functional contact and suggest that, by having different functions, the two integrins are able to cooperate but only in the presence of antigen.
To determine whether VCAM-1 assists B-cell attachment when the affinity of the BCR for antigen was much higher than for p31, we loaded lipid bilayers with a high-affinity lysozyme mutant HELRD and measured adhesion of MD4 naive B cells under shear stress (Table I). As observed with p31 and 3-83 B cells, VCAM-1 synergized with HELRD to induce more efficient binding of MD4 B cells. However, this was observed only at low antigen density (Figure 2D): equally high numbers of attached B cells were detected at high densities of antigen in the presence or absence of integrin ligands. This indicates that B cells reach an avidity above which VLA-4 binding does not contribute markedly to B-cell capture and attachment. Thus, as we previously showed for ICAM-1 (Carrasco et al, 2004), these results indicate that VCAM-1 is more efficient at increasing adhesion when the total avidity of the cell for the membrane is low.
VLA-4/VCAM-1 interaction facilitates B-cell activation
It is notable that VCAM-1 was particularly effective in mediating tethering of B cells to a target membrane and this could facilitate antigen recognition. To investigate this, we compared the frequency of B cells that raise their intracellular calcium levels when perfused, at low shear stress (0.1–0.05 dyn/cm2), over membranes containing different combinations of VCAM-1, ICAM-1 and antigen. As judged by the number of B cells that showed a calcium response, antigen-bearing membranes in the presence of VCAM-1 were far better at activating B cells than those containing ICAM-1 or antigen alone (Figure 3A and B, left panel). In contrast, no calcium flux was evident in B cells tethered to membranes in the absence of antigen. Interestingly, analysis of the magnitude of calcium response on single cells revealed a more robust signal when B cells recognized antigen in the presence of VCAM-1 than in its absence (Figure 3C, left panel). This calcium response was sufficient to fully activate the B cells, as demonstrated by the upregulation of CD86 or CD69 (Figure 3D).
Figure 3.
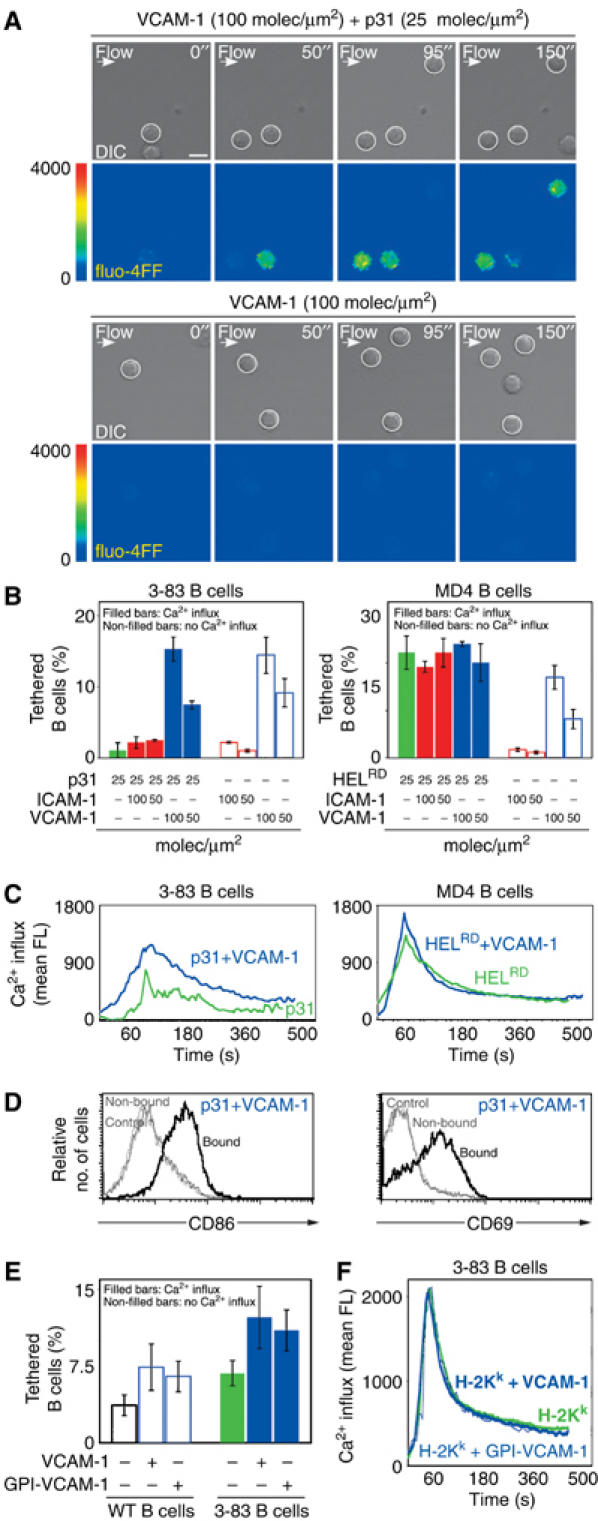
VLA-4/VCAM-1-mediated tethering enhances B-cell activation under low shear stress. Naive B cells were perfused at low shear stress (0.1–0.05 dyn/cm2) over planar membranes containing VCAM-1 or ICAM-1 in the presence or absence of antigen. (A) Different time points of two representative experiments are shown. The upper panel in each horizontal pair shows DIC images in which tethered B cells are highlighted with a white circle. The bottom panels show their calcium response as fluo-4FF fluorescence in false colour scale. Scale bar, 5 μm. (B) The frequency of tethered B cells is represented as a percentage of the total number of perfused B cells under the different conditions analysed. The left panel shows 3-83 naive B cells on membranes loaded with the indicated densities of low-affinity p31 antigen, VCAM-1 or ICAM-1. The right panel shows the same but using MD4 B cells and HELRD as high-affinity antigen. The proportion of activated B cells was determined based on the increased levels of fluo-4FF fluorescence (filled bars: activated B cells; non-filled bars: non-activated B cells). (C) The fluo-4FF fluorescence intensity was measured every 4 s for at least 50 cells in random fields. The average intensity was plotted as a function of time for 3-83 B cells (left panel) and MD4 B cells (right panel) upon tethering on antigen-bearing membranes containing p31 and HELRD respectively, in the presence (blue line) or absence (green line) of VCAM-1. (D) Activation of the 3-83 naive B cells tethered (bound) versus the non-tethered B cells (non-bound) on membranes containing VCAM-1 and p31 as antigen. B-cell activation was evaluated after 24 h as the percentage of cells that upregulate CD86 and CD69, as described in Materials and methods. (E) As in (B), percentage of WT naive B cells or 3-83 naive B cells, which recognize H2-Kk as antigen, that tether and/or raise their calcium levels over L cells (VCAM-1+/H2-Kk), DCEK (VCAM-1−/H2-Kk) or DCEK transfectants (GPI-VCAM-1+/H2-Kk). (F) As in (C), calcium response of 3-83 B cells under the conditions specified in (E).
These results indicate two mechanisms by which the interaction of VLA-4 with VCAM-1 might facilitate B-cell activation: firstly, by mediating B-cell tethering to the target membrane and thereby facilitating BCR/antigen engagement; and secondly, VLA-4 interaction with VCAM-1 appears to synergize with the BCR to enhance the extent of the B-cell signalling, either by allowing more BCR engagement or by an independent signal through VLA-4. However, this role of VCAM-1 in increasing B-cell activation seems to operate only when the BCR affinity for the antigen was low. Indeed, when a high-affinity antigen (HELRD; Table I) was loaded on the membranes, no significant difference in the percentage of B cells that sustained a calcium signal or in the strength of their response was observed either in the presence or absence of integrin ligands (Figure 3B and C, right panels). These results indicate that VLA-4 enhances BCR signalling by providing further adhesion, not by providing an independent calcium response.
It was important to ascertain whether the ability of VCAM-1 to promote B-cell activation was also functional in the more complex and physiological context of a cell surface. To this end, we initially compared the capacity of naive B cells to tether onto the surface of L cells, which express high levels of VCAM-1, or DCEK, a derived clone that lacks VCAM-1 expression. As shown in Figure 3E, naive WT B cells were significantly better at tethering on the surface of VCAM-1-expressing L cells than on DCEK cells. We then asked whether this increased tethering could promote B-cell activation. For these experiments, WT B cells were replaced with 3-83 transgenic B cells, as their BCR binds with high affinity to the H-2Kk molecule, which is endogenously expressed on the surface of both cell lines. Higher numbers of transgenic B cells showed calcium flux when bound to L cells expressing VCAM-1 than on the DCEK cell line (Figure 3E); nonetheless, and probably owing to the high affinity of the 3-83 BCR for the H-2Kk protein, no difference in the magnitude of the calcium response was observed (Figure 3F). A similar increase in B-cell tethering and degree of calcium signal was also observed when the GPI version of VCAM-1, used in the bilayer experiments, was expressed on the surface of DCEK (Figure 3E and F). In conclusion, and similar to the results obtained in the bilayer experiments, VCAM-1 can promote B-cell tethering and thereby facilitate antigen recognition.
VLA-4 can drive the recruit of GPI-VCAM-1 to the cSMAC of the B-cell synapse
The IS is a general molecular structure for antigen recognition. Therefore, we wondered whether the contribution of VLA-4 to membrane antigen recognition was accompanied by localization at the synapse. To examine its recruitment and accumulation in the B-cell synapse, we allowed naive 3-83 B cells to interact with lipid bilayers that contained GPI-anchored Alexa 543-conjugated VCAM-1 (blue), GPI-anchored Alexa 488-conjugated ICAM-1 (red) and Alexa 633-conjugated p31 (green) under static conditions. Synapse formation was then visualized by confocal fluorescence microscopy. Upon B-cell contact, ICAM-1 and antigen (p31) could be found segregated within the interface, forming a classical mature IS. Surprisingly, in all the synapses analysed, VCAM-1 mainly localized together with the engaged antigen in the central cluster (Figure 4A). Whereas ICAM-1 and antigen were engaged with similar fast kinetics in the absence (Carrasco et al, 2004) or presence of VCAM-1, it took several minutes for VCAM-1 to reach equilibrium (Figure 4B). The final density (∼300 molec/μm2) and total number of VCAM-1 molecules engaged (∼1200 molecules; Figure 4B) within the central cluster of the IS were similar in all cells analysed. As shown in Figure 4D and Supplementary Figure 2A, altering the affinity of the BCR for the antigen present in the bilayer did not alter the distribution of VCAM-1. Quantitative analysis revealed that, independent of the amount of antigen present in the bilayer, similar quantities of VCAM-1 were recruited in all cases (Figure 4E and Supplementary Figure 2B). However, the engagement of the BCR was essential, as no recruitment of VCAM-1 was observed with p0, a peptide for which the affinity of this BCR is below the threshold of B-cell activation. Furthermore, preincubation of B cells with blocking anti-VLA-4 monoclonal antibodies showed that the recruitment of VCAM-1 to the IS was indeed completely dependent on VLA-4 (Figure 4D and Supplementary Figure 2A). Thus, these results suggested that VCAM-1 can be recruited to the cSMAC of the IS by VLA-4 via a mechanism that was dependent on BCR engagement. They also indicate that VLA-4 follows a different pattern of aggregation than LFA-1.
Figure 4.
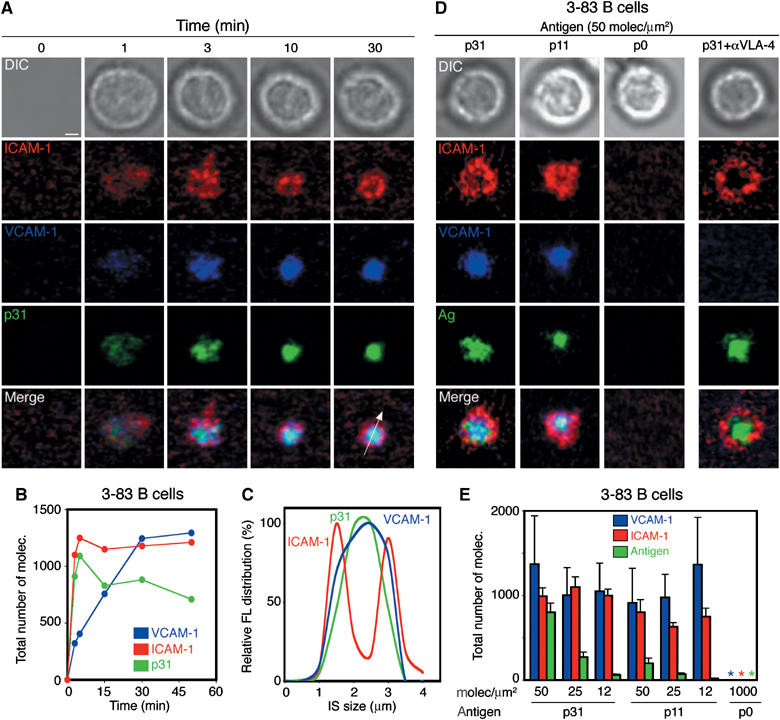
VLA-4 can drive the recruitment of GPI-VCAM-1 to the cSMAC of the IS. Naive 3-83 B cells were settled onto planar lipid bilayers containing GPI-linked ICAM-1 (red) at 50 molec/μm2, GPI-linked VCAM-1 (blue) at 25 molec/μm2 and p31 antigen (green) at 50 molec/μm2. (A) Time-lapse fluorescent images show the accumulation of p31 (green), ICAM-1 (red) and VCAM-1 (blue) in the pattern of a mature synapse at the specified time points. The top panels show DIC images at the same time points. (B) Quantification of the total number of molecules of VCAM-1, ICAM-1 and p31 antigen during synapse formation of a representative 3-83 B cell. (C) Distribution pattern of the antigen ICAM-1 and VCAM-1 fluorescent signal across a section of the IS, shown as a white arrow in (A), at 30 min. (D) Naive 3-83 B cells were settled onto membranes containing ICAM-1 (red), VCAM-1 (blue) (50 and 25 molec/μm2, respectively) and different antigens (green) at 50 molec/μm2: p31 (KA=6.5 × 107 M−1), p11 (KA∼7 × 106 M−1) and p0 (null). DIC, fluorescence and merged images from a representative B cell are shown. (E) Quantification of the number of molecules of VCAM-1, ICAM-1 and antigen recruited in the B-cell synapse shown in (D). Data represent the mean of 50 cells in each case. Scale bar, 1 μm.
VCAM-1 is part of the B-cell-antigen-presenting cell docking structure
While these observations shed light on the favoured localization of VLA-4 in the cSMAC, they do not provide any insight as to whether the association of VCAM-1 with other proteins of the target cell may control its distribution in the IS. To this end, we studied the interaction of naive 3-83 B cells with L cells expressing a GFP-linked full-length VCAM-1 as antigen-presenting cells. In parallel, the BCR localization was assessed by staining B cells with non-blocking fluorescently labelled Fab fragments against IgM, before antigen exposure. Three-dimensional reconstruction of confocal images showed that, in at least 90% of the conjugates analysed, VCAM-1-GFP participated in the formation of a docking structure surrounding the BCR/antigen and was completely excluded from the cSMAC (Figure 5A and B and Supplementary Movie S2). Furthermore, these observations were confirmed by immunostaining of the endogenous VCAM-1 expressed by the L cells, which showed a similar pattern of distribution (data not shown). Interestingly, with target cells expressing ICAM-1-GFP, we observed a good colocalization of the two integrin ligands in the cup structure (Figure 5C). These results showed the participation of VCAM-1 in the docking structure that we previously described for the B-cell synapse (Carrasco et al, 2004). Furthermore, they also indicate that its localization in the IS was not simply the result of the interaction with VLA-4, but it was likely to involve association of VCAM-1 with other proteins in the target cell.
Figure 5.
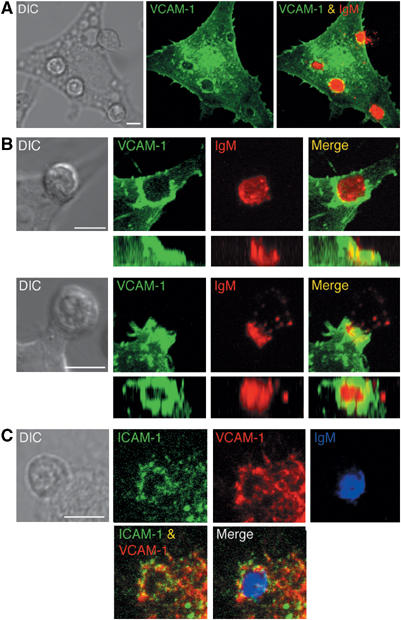
Full VCAM-1 localizes in the periphery of the B-cell synapse. (A) Interaction in real time of naive 3-83 B cells stained with Cy5-conjugated non-blocking anti-IgM Fabs (red) with L cells expressing VCAM-1-GFP (green) was analysed by confocal microscopy. Separate and merged three-dimensional projections of the confocal fluorescence image stacks taken after 20 min incubation are shown. DIC image at the same time point is shown in the left panel. (B) Side and top views of the three-dimensional reconstruction of the docking structure formed by VCAM-1-GFP in the B-cell synapse. Two different examples of DIC, separate and merged fluorescent images of VCAM-1-GFP and IgM are shown. (C) 3-83 B cells interacting with ICAM-1-GFP target cells were fixed, permeabilized and stained for endogenous VCAM-1 and IgM with anti-VCAM-1 (red) and anti-IgM antibodies (blue). DIC, separate and merged three-dimensional projection of the confocal fluorescence image stacks of VCAM-1, ICAM-1 and IgM are shown. Scale bar, 5 μm.
Localization of VCAM-1 in the docking structure and its exclusion from the cSMAC are dependent on its transmembrane domain
The fact that full-length VCAM-1 was recruited and segregated into the pSMAC of the IS when expressed on target cells, but the GPI-VCAM-1 used in the lipid bilayer was recruited but not properly segregated, suggested that its proper segregation relies upon the transmembrane domain and/or cytoplasmic tail. If this interpretation was correct, one would expect that a truncated form of VCAM-1 expressed on the surface of target cells should fail to be distributed to the pSMAC. As shown in Figure 6A, this was indeed the case. Mislocalization represented as lack of exclusion from the cSMAC was observed when synapse formation was analysed with target cells that expressed either a truncated form of VCAM-1, in which its extracellular part has been linked to the H-2Kb transmembrane domain and a short intracytoplasmic tail (EC VCAM-1/H2), or GPI-VCAM-1 (Figure 6A and B). In contrast, an intracytoplasmic tailless version of this molecule (VCAM-1 tailless-GFP) was excluded, which indicates that the transmembrane domain of the protein was responsible for the proper localization in the IS. Support for this observation also came from studying the distribution of the different VCAM-1 mutants on the target cells. It was noticeable that full-length VCAM-1-GFP was usually expressed on the surface of microvilli (Figure 6C and D). In contrast, GPI-VCAM-1 or EC VCAM-1/H2 was distributed evenly across the membrane, indicating that these forms are differentially associated with the cytoskeleton of the target cells. Finally, treating target cells with inhibitors of actin polymerization, like cytochalasin D, disrupted VCAM-1-GFP's microvilli distribution (data not shown). These results suggest that VCAM-1 is linked to the actin cytoskeleton via the interaction of its transmembrane domain with another protein. While, as shown in Figure 3, the link between VCAM-1 and the cytoskeleton of the target cell did not seem to be highly significant in mediating B-cell tethering and facilitating activation, it appeared to be a requirement for the exclusion of VCAM-1 from the cSMAC and the sorting of this molecule in the cup-like structure that characterizes the B-cell IS.
Figure 6.
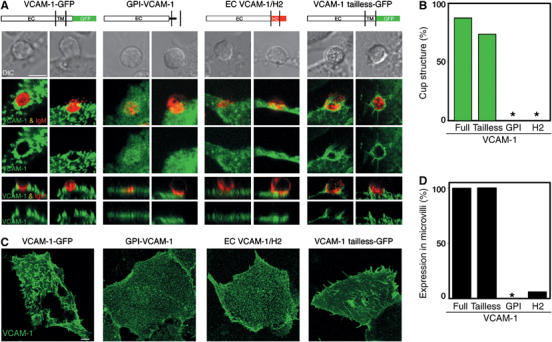
The transmembrane domain of VCAM-1 determines its distribution in the IS. (A) Interaction of 3-83 naive B cells with DCEK cells transfected with the different VCAM-1 constructs. 3-83 B cells interacting with DCEK cells transfected with GPI-VCAM-1 or EC VCAM-1/H2 were fixed, permeabilized and stained with antibodies against VCAM-1 and IgM, as indicated in Materials and methods. 3-83 B cells, previously stained with Cy5-conjugated non-blocking anti-IgM Fabs, were in contact with VCAM-1-GFP and VCAM-1 tailless-GFP DCEK transfectants for 25 min at 37°C. Then, confocal life-imaging was acquired. Two different examples are shown for each VCAM-1 construct. The middle panels show top views of fluorescent images representing a projection of several horizontal confocal sections. The bottom panels show a sagittal section of the top view in each case. DIC images are shown in the top panel. Schematic illustrations of the different VCAM-1 constructs are shown on top of the corresponding panels. Scale bar, 5 μm. (B) The four VCAM-1 constructs were evaluated for their capacity to be excluded and form a docking structure in the B-cell synapse. Data obtained from at least 30 B-cell synapses were analysed in each case. (C) COS transfectants of the different VCAM-1 constructs were fixed and stained with anti-VCAM-1 mAb (green). Each panel represents a projection of the confocal fluorescence image stack. Scale bar, 5 μm. (D) The four VCAM-1 constructs were evaluated for their capacity to be expressed on microvilli at the surface of DCEK cells. Data obtained from at least 30 DCEK transfected cells were analysed in each case.
Discussion
This study describes the role of VLA-4/VCAM-1 interaction during B-cell recognition of membrane-bound antigens. We find that, under static conditions in synergy with the BCR, VLA-4 enhances B-cell adhesion to an antigen-bearing membrane. We also show that, under low shear stress, VCAM-1 becomes a prerequisite for antigen recognition, suggesting that this adhesion pair is involved during the first stages of the interaction between the B cell and the target membrane. Furthermore, we establish that, when the affinity of the BCR for the antigen is low, the interaction of VLA-4 with VCAM-1 facilitates B-cell activation. This is achieved by two independent mechanisms: by mediating B-cell tethering to the target membrane and thereby facilitating antigen/BCR engagement, and by enhancing the level of signalling.
It is likely that this increase in B-cell signalling is mainly owing to the capacity of VCAM-1 to promote tight adhesion. As a consequence of this, more antigen engagement of the BCR might occur. This presumption is based on our experimental observation, which indicates that the enhanced adhesion by VLA-4/VCAM-1 allows better antigen gathering under static conditions (Figure 1 and data not shown), as previously described for the LFA-1/ICAM-1 interaction (Carrasco et al, 2004). Furthermore, no difference in the levels of calcium response can be detected when the affinity of the BCR for the antigen was high. However, at this point, it is not possible to completely rule out whether VLA-4 is able to deliver an independent signal that could account for the increased calcium response or may even follow a calcium-independent pathway. In this line, it has been shown that the interaction of VLA-4 with VCAM-1 can protect germinal centre or peripheral blood B cells from apoptosis (Koopman et al, 1994; Hayashida et al, 2000). In the latter case, this seems to be mediated by an upregulation of Bcl-XL.
We also provide evidence that the ectodomain of VCAM-1 alone is sufficient to perform these functions in the absence of any linkage with the cytoskeleton of the target cell. Using the planar bilayers system, we find that a GPI version of VCAM-1 is sufficient to mediate B-cell tethering and enhance signalling. Similarly, expression of the GPI-VCAM-1 on the surface of target cells can also promote B-cell activation as well as a full VCAM-1 molecule. These results are intriguing as the topological distribution of selectins and adhesion molecules in the microvilli of leucocytes has been shown to be critical in enhancing their tethering capacity (von Andrian et al, 1995). However, it is important to point out that this increase in tethering was observed only under shear rates that prevail in microvessels, which are much higher than those utilized in our study. Indeed, differences in adhesion, depending on the topological distribution, were not detected under static conditions. Furthermore, and in line with our results, it has been shown that firm adhesion of leucocytes mediated by VLA-4 and LFA-1 is not affected by the mislocalization of their respective ligands, VCAM-1 and ICAM-1, due to the disruption of the endothelium cytoskeleton (Carman et al, 2003).
The role of VCAM-1 in promoting antigen recognition seems to be independent of its final localization at the IS. Thus, this function in enhancing B-cell activation is likely to precede the sorting of the molecule in the IS. Upon antigen recognition, VCAM-1 segregates to form a docking structure that is characteristic of the B-cell IS. The transmembrane domain of the protein determines this localization. This conclusion is based on our lipid bilayer experiments where a GPI-VCAM-1 form is recruited to the cSMAC by VLA-4. Furthermore, in cell-to-cell interaction experiments, EC VCAM-1/H2 and GPI-VCAM-1, but not VCAM-1 tailless-GFP, fail to be excluded from the cSMAC. Interestingly, the group of Sanchez-Madrid (Barreiro et al, 2005) has recently shown that VCAM-1 is involved in the interaction with tetraspanin proteins that probably link VCAM-1 and ICAM-1 on the surface of a target cell. In a previous study, these authors showed that the same two integrin ligands participate in the formation of a ‘cup-like' structure that anchors leucocytes to the endothelium (Barreiro et al, 2002). In this structure, ICAM-1 and VCAM-1 appear to be linked to the actin cytoskeleton by the association with ERM proteins. In addition, the group of Springer has shown that ICAM-1 and VCAM-1 are associated with actin-rich microvilli in the ‘cup-like' structure and has suggested that this provides directional guidance to leucocytes for extravasation (Carman et al, 2003; Carman and Springer, 2004). Both groups speculate that such a structure facilitates leucocyte migration. This study and previous evidence (Carrasco et al, 2004) show that a similar structure is formed in the context of the B-cell synapse. This docking structure is likely to play a role in providing firm adhesion to the central cluster of BCR/antigen and we suggest that, in this context, it may facilitate antigen extraction for presentation to T cells.
The peripheral localization of LFA-1/ICAM-1 is one of the characteristic features of the IS (Monks et al, 1998; Wulfing et al, 1998; Grakoui et al, 1999). The retention of LFA-1 in the pSMAC seems to be mediated by its association with the cytoskeleton through talin (Monks et al, 1998). Our results suggest that VLA-4 follows an inverse pattern of distribution. Consistent with this notion, it has recently been shown that, in the presence of blocking antibodies, VLA-4 also localizes in the cSMAC of the CD4 T cell (Mittelbrunn et al, 2004); however, this was observed in the absence of detectable VCAM-1. These lines of evidence strongly argue that VLA-4 is likely to associate differently from LFA-1 with the cell cytoskeleton. Such a differential association may well relate with an overlapping but sequential function of VLA-4 and LFA-1.
Several studies using T cells have highlighted the capacity of VLA-4 to mediate tethering and strong adhesion within the multistep model of leucocyte trafficking (Butcher, 1991; Springer, 1994; Alon et al, 1995; Berlin et al, 1995; Grabovsky et al, 2000). In this model, the first step involves transient and reversible adhesion that, when followed by a second event, results in strong and sustained attachment owing to the activation of integrins. Chemokines have been proposed as the mediators of the second step; however, a possible role for other receptors was anticipated. Indeed, recently, FcγRIII has been shown to mediate leucocyte adhesion in response to ICs in vivo and in vitro (Coxon et al, 2001; Stokol et al, 2004). It is conceivable that the BCR may play a similar role in the recruitment and activation of B cells. In this line, VCAM-1 is highly expressed on the surface of FDCs where it has been shown to enhance the interaction with B cells (Freedman et al, 1990; Koopman et al, 1991). Similarly, a role for VLA-4 in promoting antigen recognition may well be important in determining B-cell recruitment and activation in pathological situations such as in rheumatoid arthritis, systemic lupus erythematosus and glomerulonephritis. These diseases are characterized by the accumulation of ICs in cartilage surface (Matsumoto et al, 2002), synovial arterioles (Schaller et al, 2001) and postcapillary venules (Smedegard et al, 1985), together with high levels of expression of adhesion molecules, such as VCAM-1 (van Dinther-Janssen et al, 1991; Belmont et al, 1994).
Thus, incorporating our results into a model of B-cell membrane antigen recognition (Figure 7), VCAM-1 expressed on the surface of target cells (FDC, vascular endothelium) may capture the B cell through its interaction with VLA-4 under conditions of restrictive/limiting cellular interactions (germinal centre, shear stress). The absence of antigen recognition will result in B-cell detachment from the target cell within seconds. However, the encounter of specific antigen by the BCR will result in inside-out signalling to VLA-4 and LFA-1 in order to strengthen the interaction with VCAM-1 and promote the interaction with ICAM-1, respectively. This firm attachment to the target cell would allow the B cell to form a mature IS and become activated. This model may well apply to autoimmune disorders, in which the chronic expression of VCAM-1 could facilitate B-cell triggering by low-affinity self-antigens.
Figure 7.
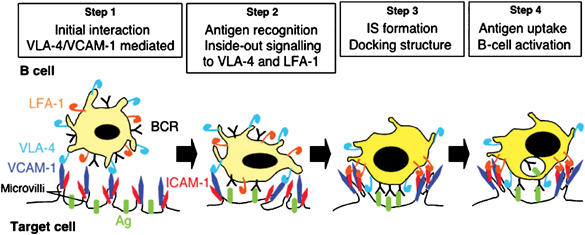
Model of B-cell membrane antigen recognition.
Materials and methods
Mice and splenic B-cell purification
Three different mouse strains were used: C57BL/6 WT mice and the BCR transgenic lines MD4 (HEL-specific BCR; Goodnow et al, 1988) and 3-83 (H-2Kk-specific BCR; Russell et al, 1991). Splenic naive B cells were purified by negative selection, as previously described (Carrasco et al, 2004). An enrichment of 95–98% B cells was obtained by this procedure.
3-83 peptide ligands and recombinant lysozymes
The three different monobiotinylated peptide ligands (p31, p11 and p0) and the lysozyme mutants (HELRD, with amino-acid positions 21 and 101 mutated to alanine; HELKD, with amino-acid positions 97 and 101 mutated to alanine; and HELRKD, with amino-acid positions 21, 97 and 101 mutated to alanine; Batista and Neuberger, 1998) used in this study were obtained as described previously (Carrasco et al, 2004).
Generation of VCAM-1 constructs
The GPI-linked VCAM-1 construct was generated as follows. The extracellular domain of murine VCAM-1 was amplified by PCR with cDNA obtained from murine 3T3 NIH fibroblast as a template, using the sense primer 5′-GGGGTACCCAGAGACTTGAAATGCCTG-3′ and the antisense primer 5′-GCGAATTCCACTACCTGAATAGTCCTTGTTAT GTTCTTTTCC-3′. The fragment obtained was cloned into KpnI/EcoRI restriction sites on pcDNA3 expression vector containing the GPI domain coding sequence (Carrasco et al, 2004). The GPI-linked VCAM-1 construct was transfected into J558L cells and positive clones were selected in the presence of geneticin.
The complete murine VCAM-1 and VCAM-1 tailless coding sequences were amplified using the same sense primer 5′-TCCCCCCGGGCGCTTCCACCTCCACTCACTTT GGATTTCTGTGC-3′ and antisense primer 5′-TCCCCCCGGGAAGCTTTTCTTGCAAAATAAAC -3′ and then cloned into KpnI/XmaI in the pEGFP-N1 expression vector. The EC VCAM-1/H2 construct was generated by replacement of the coding sequence of the GPI domain in the GPI-linked VCAM-1 construct with the coding sequence of a Ser/Gly linker, plus the H-2Kb transmembrane region, plus a cytoplasmic tail obtained from a lysozyme construct described by Batista et al (2001).
Planar lipid bilayers and laminar shear stress assays
The planar lipid bilayers containing the different proteins were prepared as previously described (Grakoui et al, 1999; Carrasco et al, 2004). The chambers were blocked with PBS 2% FCS, followed by antigen loading in PBS 0.5% FCS. The different antigens for 3-83 and MD4 B cells were loaded in the bilayers using Alexa 633-streptavidin (Molecular Probes), as previously described (Carrasco et al, 2004). For lamina shear stress assays, the flow chamber was connected to a pump in order to control the shear stress applied and B cells were perfused at 1 × 106/ml. For the Ca2+ measurements, B cells were previously stained with 1 μm fluo-4FF (Molecular Probes). The assays were performed in PBS 0.5% FCS, 0.5 mM Mg2+, 0.5 mM Ca2+ and 1 g/l D-glucose at 37°C. A series of three DIC images, with a second delay between each, were taken every minute during all shear stress experiments. Images were acquired on a Zeiss Axiovert LSM 510-META inverted microscope and analysed by Volocity (Improvision, UK) and ImageReady (Adobe) softwares. The number of B cells bound per mm2 was calculated by counting the B cells that remain in the same position on the three images of each series. The fraction of B cells bound, Ca2+ fluxing or rolling was estimated by counting these events within the total number of B cells flowing. For the activation assay, bound and not bound fractions of B cells were incubated overnight inside the flow chamber and in a dish, respectively. Then, they were collected and analysed by FACS for the expression of CD86 and CD69. The shear stress applied in dyn/cm2 was calculated by the Hagen–Poiseuille equation.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Movie S1
Supplementary Movie S2
Acknowledgments
We thank Michael R Ehrenstein, Nancy Hogg, Alison McDowall, Martijn A Nolte, David Sancho-Madrid, Violeta Silva-Vargas, Andrew Smith, Caetano Reis e Sousa and Michael Way for helpful discussions and critical reading of the manuscript. This work was funded by Cancer Research UK, an EMBO Long Term Fellowship (YRC) and an EMBO YIP Award (FDB).
References
- Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA (1995) The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol 128: 1243–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton A, Born GV (1972) Quantitative investigations of the adhesiveness of circulating polymorphonuclear leucocytes to blood vessel walls. J Physiol 222: 447–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro O, Yanez-Mo M, Sala-Valdes M, Gutierrez-Lopez MD, Ovalle S, Higginbottom A, Monk PN, Cabanas C, Sanchez-Madrid F (2005) Endothelial tetraspanin microdomains regulate leukocyte firm adhesion during extravasation. Blood 105: 2852–2861 [DOI] [PubMed] [Google Scholar]
- Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F (2002) Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol 157: 1233–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista FD, Neuberger MS (1998) Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity 8: 751–759 [DOI] [PubMed] [Google Scholar]
- Batista FD, Iber D, Neuberger MS (2001) B cells acquire antigen from target cells after synapse formation. Nature 411: 489–494 [DOI] [PubMed] [Google Scholar]
- Belmont HM, Buyon J, Giorno R, Abramson S (1994) Up-regulation of endothelial cell adhesion molecules characterizes disease activity in systemic lupus erythematosus. The Shwartzman phenomenon revisited. Arthritis Rheum 37: 376–383 [DOI] [PubMed] [Google Scholar]
- Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC (1995) alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 80: 413–422 [DOI] [PubMed] [Google Scholar]
- Butcher EC (1991) Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 67: 1033–1036 [DOI] [PubMed] [Google Scholar]
- Carman CV, Springer TA (2004) A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol 167: 377–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman CV, Jun CD, Salas A, Springer TA (2003) Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J Immunol 171: 6135–6144 [DOI] [PubMed] [Google Scholar]
- Carrasco YR, Fleire SJ, Cameron T, Dustin ML, Batista FD (2004) LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity 20: 589–599 [DOI] [PubMed] [Google Scholar]
- Chen C, Mobley JL, Dwir O, Shimron F, Grabovsky V, Lobb RR, Shimizu Y, Alon R (1999) High affinity very late antigen-4 subsets expressed on T cells are mandatory for spontaneous adhesion strengthening but not for rolling on VCAM-1 in shear flow. J Immunol 162: 1084–1095 [PubMed] [Google Scholar]
- Chigaev A, Zwartz G, Graves SW, Dwyer DC, Tsuji H, Foutz TD, Edwards BS, Prossnitz ER, Larson RS, Sklar LA (2003) Alpha4beta1 integrin affinity changes govern cell adhesion. J Biol Chem 278: 38174–38182 [DOI] [PubMed] [Google Scholar]
- Coxon A, Cullere X, Knight S, Sethi S, Wakelin MW, Stavrakis G, Luscinskas FW, Mayadas TN (2001) Fc gamma RIII mediates neutrophil recruitment to immune complexes. A mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity 14: 693–704 [DOI] [PubMed] [Google Scholar]
- Freedman AS, Munro JM, Rice GE, Bevilacqua MP, Morimoto C, McIntyre BW, Rhynhart K, Pober JS, Nadler LM (1990) Adhesion of human B cells to germinal centers in vitro involves VLA-4 and INCAM-110. Science 249: 1030–1033 [DOI] [PubMed] [Google Scholar]
- Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, Trent RJ, Basten A (1988) Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature 334: 676–682 [DOI] [PubMed] [Google Scholar]
- Grabovsky V, Feigelson S, Chen C, Bleijs DA, Peled A, Cinamon G, Baleux F, Arenzana-Seisdedos F, Lapidot T, van Kooyk Y, Lobb RR, Alon R (2000) Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J Exp Med 192: 495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML (1999) The immunological synapse: a molecular machine controlling T cell activation. Science 285: 221–227 [DOI] [PubMed] [Google Scholar]
- Haberman AM, Shlomchik MJ (2003) Reassessing the function of immune-complex retention by follicular dendritic cells. Nat Rev Immunol 3: 757–764 [DOI] [PubMed] [Google Scholar]
- Hayashida K, Shimaoka Y, Ochi T, Lipsky PE (2000) Rheumatoid arthritis synovial stromal cells inhibit apoptosis and up-regulate Bcl-xL expression by B cells in a CD49/CD29–CD106-dependent mechanism. J Immunol 164: 1110–1116 [DOI] [PubMed] [Google Scholar]
- Koopman G, Keehnen RM, Lindhout E, Newman W, Shimizu Y, van Seventer GA, de Groot C, Pals ST (1994) Adhesion through the LFA-1 (CD11a/CD18)–ICAM-1 (CD54) and the VLA-4 (CD49d)–VCAM-1 (CD106) pathways prevents apoptosis of germinal center B cells. J Immunol 152: 3760–3767 [PubMed] [Google Scholar]
- Koopman G, Parmentier HK, Schuurman HJ, Newman W, Meijer CJ, Pals ST (1991) Adhesion of human B cells to follicular dendritic cells involves both the lymphocyte function-associated antigen 1/intercellular adhesion molecule 1 and very late antigen 4/vascular cell adhesion molecule 1 pathways. J Exp Med 173: 1297–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosco-Vilbois MH (2003) Are follicular dendritic cells really good for nothing? Nat Rev Immunol 3: 764–769 [DOI] [PubMed] [Google Scholar]
- Kouskoff V, Famiglietti S, Lacaud G, Lang P, Rider JE, Kay BK, Cambier JC, Nemazee D (1998) Antigens varying in affinity for the B cell receptor induce differential B lymphocyte responses. J Exp Med 188: 1453–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel MF, Sjaastad MD, Wulfing C, Davis MM (2000) Differential clustering of CD4 and CD3zeta during T cell recognition. Science 289: 1349–1352 [DOI] [PubMed] [Google Scholar]
- Lang J, Jackson M, Teyton L, Brunmark A, Kane K, Nemazee D (1996) B cells are exquisitely sensitive to central tolerance and receptor editing induced by ultralow affinity, membrane-bound antigen. J Exp Med 184: 1685–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A (1985) Antigen-specific interaction between T and B cells. Nature 314: 537–539 [DOI] [PubMed] [Google Scholar]
- Leuker CE, Labow M, Muller W, Wagner N (2001) Neonatally induced inactivation of the vascular cell adhesion molecule 1 gene impairs B cell localization and T cell-dependent humoral immune response. J Exp Med 193: 755–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CG, Lu TT, Cyster JG (2003) Integrin-dependence of lymphocyte entry into the splenic white pulp. J Exp Med 197: 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lollo BA, Chan KW, Hanson EM, Moy VT, Brian AA (1993) Direct evidence for two affinity states for lymphocyte function-associated antigen 1 on activated T cells. J Biol Chem 268: 21693–21700 [PubMed] [Google Scholar]
- Lu TT, Cyster JG (2002) Integrin-mediated long-term B cell retention in the splenic marginal zone. Science 297: 409–412 [DOI] [PubMed] [Google Scholar]
- Matsumoto I, Maccioni M, Lee DM, Maurice M, Simmons B, Brenner M, Mathis D, Benoist C (2002) How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nat Immunol 3: 360–365 [DOI] [PubMed] [Google Scholar]
- McLeod SJ, Li AH, Lee RL, Burgess AE, Gold MR (2002) The Rap GTPases regulate B cell migration toward the chemokine stromal cell-derived factor-1 (CXCL12): potential role for Rap2 in promoting B cell migration. J Immunol 169: 1365–1371 [DOI] [PubMed] [Google Scholar]
- Mittelbrunn M, Molina A, Escribese MM, Yanez-Mo M, Escudero E, Ursa A, Tejedor R, Mampaso F, Sanchez-Madrid F (2004) VLA-4 integrin concentrates at the peripheral supramolecular activation complex of the immune synapse and drives T helper 1 responses. Proc Natl Acad Sci USA 101: 11058–11063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, Medina K, Ishihara K, Kimoto M, Auerbach R, Kincade PW (1991) A VCAM-like adhesion molecule on murine bone marrow stromal cells mediates binding of lymphocyte precursors in culture. J Cell Biol 114: 557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A (1998) Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395: 82–86 [DOI] [PubMed] [Google Scholar]
- Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R (1989) Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 59: 1203–1211 [DOI] [PubMed] [Google Scholar]
- Russell DM, Dembic Z, Morahan G, Miller JF, Burki K, Nemazee D (1991) Peripheral deletion of self-reactive B cells. Nature 354: 308–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M, Burton DR, Ditzel HJ (2001) Autoantibodies to GPI in rheumatoid arthritis: linkage between an animal model and human disease. Nat Immunol 2: 746–753 [DOI] [PubMed] [Google Scholar]
- Smedegard G, Bjork J, Arfors KE (1985) An intravital microscopy model for studies of immune complex induced inflammation at the microvascular level. Int J Tissue React 7: 55–60 [PubMed] [Google Scholar]
- Spaargaren M, Beuling EA, Rurup ML, Meijer HP, Klok MD, Middendorp S, Hendriks RW, Pals ST (2003) The B cell antigen receptor controls integrin activity through Btk and PLCgamma2. J Exp Med 198: 1539–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA (1990) Adhesion receptors of the immune system. Nature 346: 425–434 [DOI] [PubMed] [Google Scholar]
- Springer TA (1994) Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76: 301–314 [DOI] [PubMed] [Google Scholar]
- Stokol T, O'Donnell P, Xiao L, Knight S, Stavrakis G, Botto M, von Andrian UH, Mayadas TN (2004) C1q governs deposition of circulating immune complexes and leukocyte Fcgamma receptors mediate subsequent neutrophil recruitment. J Exp Med 200: 835–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakal AK, Kosco MH, Tew JG (1988) FDC-iccosome mediated antigen delivery to germinal center B cells, antigen processing and presentation to T cells. Adv Exp Med Biol 237: 197–202 [DOI] [PubMed] [Google Scholar]
- van Dinther-Janssen AC, Horst E, Koopman G, Newmann W, Scheper RJ, Meijer CJ, Pals ST (1991) The VLA-4/VCAM-1 pathway is involved in lymphocyte adhesion to endothelium in rheumatoid synovium. J Immunol 147: 4207–4210 [PubMed] [Google Scholar]
- von Andrian UH, Hasslen SR, Nelson RD, Erlandsen SL, Butcher EC (1995) A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell 82: 989–999 [DOI] [PubMed] [Google Scholar]
- Wulfing C, Sjaastad MD, Davis MM (1998) Visualizing the dynamics of T cell activation: intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc Natl Acad Sci USA 95: 6302–6307 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Movie S1
Supplementary Movie S2


