Abstract
The protein Sup35 from Saccharomyces cerevisiae possesses prion properties. In vivo, a high molecular weight form of Sup35p is associated to the [PSI+] factor. The continued propagation of [PSI+] is highly dependent on the expression levels of molecular chaperones from the Hsp100, 70 and 40 families; however, so far, their role in this process is unclear. We have developed a reproducible in vitro system to study the effects of molecular chaperones on the assembly of full-length Sup35p. We show that Hsp104p greatly stimulates the assembly of Sup35p into fibrils, whereas Ydj1p has inhibitory effect. Hsp82p, Ssa1p and Sis1p, individually, do not affect assembly. In contrast, Ssa1p together with either of its Hsp40 cochaperones blocks Sup35p polymerization. Furthermore, Ssa1p and Ydj1p or Sis1p can counteract the stimulatory activity of Hsp104p, by forming complexes with Sup35p oligomers, in an ATP-dependent manner. Our observations reveal the functional differences between Hsp104p and the Hsp70–40 systems in the assembly of Sup35p into fibrils and bring new insight into the mechanism by which molecular chaperones influence the propagation of [PSI+].
Keywords: fibrils, molecular chaperones, prions, [PSI+], Sup35p
Introduction
The epigenetic factor [PSI+] in the yeast Saccharomyces cerevisiae (Cox, 1965) is due to the prion properties of the protein Sup35 (Wickner, 1994). In the prion state, insoluble aggregates of Sup35p (or eRF3, eukaryotic translation termination factor) give rise to [PSI+] cells with altered translation termination, as manifested by an increased tendency of ribosomes to read through nonsense ochre stop codons (Cox et al, 1988). ‘Weak' and ‘strong' [PSI+] variants have been described that possess different mitotic stabilities and stop codon suppression capacities, possibly as the consequence of different prion structures (Derkatch et al, 1996).
In vivo, Sup35p aggregates associated to [PSI+] have increased resistance to proteolysis, while in [psi−] cells, Sup35p is soluble and proteinase K sensitive (Patino et al, 1996; Paushkin et al, 1996). The Sup35p-containing complexes characteristic of the [PSI+] determinant are composed of Sup35p and associated proteins. These complexes vary in size in different [PSI+] variants (Kryndushkin et al, 2003).
The full-length Sup35p assembles spontaneously in vitro into protein fibrils with an average diameter of 11–17±1–2 nm that are over 1 μm long. The region critical for assembly into fibrils is the N-terminal domain extending from amino-acid residue 1 to 123 (Sup35pN). This domain alone or together with the middle domain (Sup35pNM) of the protein (amino-acid residues 124–253) forms fibrils, which exhibit the characteristics of amyloids in that they (i) have increased resistance to proteolysis, (ii) bind the dye Congo red and exhibit the 4.7 Å reflection in X-ray fiber diffraction images, and (iii) assemble in a cooperative manner, following a nucleation growth process that can be greatly facilitated with preformed fibrils, thus mimicking in a test tube the invasive propagation of [PSI+] (Glover et al, 1997; King et al, 1997; Serio et al, 2000). The majority of in vitro studies on the assembly of Sup35p have been performed using its prion-determining region (Sup35pNM) as fibrils made of Sup35pNM assembled in vitro propagate [PSI+] when reintroduced in yeast cells (King and Diaz-Avalos, 2004; Tanaka et al, 2004). However, there is no evidence that Sup35p is degraded upon its aggregation in [PSI+] cells.
In vivo, the continued propagation of yeast prions is highly dependent on the expression levels of a number of molecular chaperones (Tuite and Cox, 2003). Indeed, members of the Hsp100, Hsp70 and Hsp40 protein families modulate the propagation of [PSI+], but their roles at the molecular level are subject to debate.
The effect of Hsp104p on the propagation of [PSI+] has been actively studied, since it was shown that either inactivation or overproduction of the molecular chaperone caused the loss of [PSI+] (Chernoff et al, 1995). Hsp104p has been reported to act both on the soluble and insoluble high molecular weight forms of Sup35p in vivo. When Hsp104p activity is decreased, large aggregates of Sup35p accumulate within the cells and the faithful propagation of [PSI+] is impaired (Wegrzyn et al, 2001), while the overexpression of Hsp104p is accompanied by an increase in the amount of soluble Sup35p (Paushkin et al, 1996).
Two alternative models have been proposed to explain the effect of Hsp104p on [PSI+] propagation. As Hsp104p, in coordination with the Hsp70–40 chaperone system, is involved in the solubilization and refolding of aggregated proteins (Parsell et al, 1994; Glover and Lindquist, 1998), it was proposed that Hsp104p fragments prion particles into smaller oligomers, thus allowing their successful propagation to daughter cells upon division (Kushnirov and Ter-Avanesyan, 1998). However, the mode of action of Hsp104p in the disaggregation of heat-aggregated proteins and the potential mechanistic differences with prion aggregate fragmentation are not well characterized. Alternatively, Hsp104p is believed to be primarily required for an inherently unstable protein-folding event, necessary for prion formation (Patino et al, 1996; Serio and Lindquist, 2000).
Molecular chaperones belonging to the Hsp70 and Hsp40 families, although not strictly required, have also been shown to influence [PSI+] propagation. Mutations in the ATPase domain of Ssa1p, as well as in residues important for the interaction with Hsp40, impair [PSI+] propagation (Jung et al, 2000; Jones and Masison 2003). Unbalanced levels of overexpressed Ydj1p and Ssa1p were shown to cause prion loss in either ‘weak' native or chimeric [PSI+] strains (Kushnirov et al, 2000). While elevated levels of Ssa1p counteract [PSI+] loss upon overexpression of Hsp104p (Newnam et al, 1999), other members of the Hsp70 family, Ssb1 and Ssb2, enhance [PSI+] curing under similar conditions and influence the frequency of spontaneous [PSI+] formation in [psi−] cells (Chernoff et al, 1999).
Most studies of the role of molecular chaperones in the propagation of [PSI+] have been performed in vivo where the effects of a molecular chaperone can be counterbalanced by another chaperone. The limited in vitro studies that have been carried out employed the Sup35pNM fragment (Inoue et al, 2004; Shorter and Lindquist, 2004).
Here we document, for the first time by in vitro techniques, the effect of molecular chaperones from the Hsp40, Hsp70, Hsp90 and Hsp100 families, individually and in concert, on the assembly of the full-length Sup35p. Our observations reveal the functional differences between Hsp104p and the Hsp70–40 systems in the assembly of Sup35p into protein fibrils, provide a rationale for the effect of the different molecular chaperones on prion formation and bring new insight into the mechanism by which molecular chaperones interplay influences the propagation of [PSI+].
Results
Full-length Sup35p is a suitable substrate for prion assembly studies
Recombinant, renatured Sup35NM fragment is used in the vast majority of studies performed in vitro on the assembly of Sup35p. To make sure that the conclusions derived from our study of the interaction of molecular chaperones with Sup35p would not be subject to limitations due, in particular, to differences in the surfaces of Sup35pNM and full-length Sup35p, we chose to use soluble, full-length protein.
For this purpose, we developed a reproducible protocol for the purification of recombinant, full-length Sup35p, using nondenaturing conditions (see Materials and methods). The full-length Sup35p assembles into high molecular weight oligomers (Figure 1), as does its NM fragment (Glover et al, 1997). Three phases can be distinguished in the assembly reaction. A lag phase, where nucleation occurs, followed by an elongation phase preceding a steady state (plateau). The assembly reaction is highly cooperative as the lag phase can be shortened significantly by increasing the Sup35p concentration (Figure 1A). The nucleation phase is the limiting step in the assembly reaction since it is abolished by the addition of preformed Sup35p fibrils (Figure 1B). Fibrils obtained under our experimental conditions are long (several μm), 25 nm wide, rigid and twisted, and have a high tendency to bundle (Figure 1C). All the experiments presented in this study were performed at a protein concentration of 4–6 μM. Under these conditions, the lag phase extends over 6–8 h and a steady state is reached within 35 h.
Figure 1.

Assembly of the full-length Sup35p into protein fibrils. (A) Dependence of the lag time preceding assembly on the concentration of Sup35p. Soluble Sup35p, 4 μM (•) and 6 μM (▪) was incubated without shaking at 10°C in the assembly buffer. Aliquots were removed at regular time intervals and assayed using the thioflavin T binding assay described in the Materials and methods section. (B) The lag time preceding assembly is the rate-limiting reaction in the assembly process. Assembly of soluble Sup35p (6 μM) in the absence (•) or the presence (▴) of preformed Sup35p fibrils (1 μM). AU, arbitrary units. (C) Negative-stained electron micrographs of fibrils made from the full-length Sup35p. Bar, 0.3 μm.
Effects of individual yeast molecular chaperones on the assembly of Sup35p into high molecular weight oligomers
The effects of Hsp104p (Hsp100), Hsp82p (Hsp90), Ssa1p (Hsp70), Ydj1p and Sis1p (Hsp40) on Sup35p assembly were monitored using thioflavin T binding. Molecular chaperones were tested both in sub- and equimolar monomer concentrations (Figure 2). Hsp82p, Ssa1p and Sis1p in sub- or equimolar concentrations have no effect on Sup35p assembly (Figure 2A and B, respectively). In contrast, Ydj1p and Hsp104p strongly influenced the reaction (Figure 2C). While Ydj1p has a strong inhibitory effect, Hsp104p promotes Sup35p assembly. Indeed, the thioflavin T fluorescence intensities at steady state in the presence of Ydj1p and Sup35p at a ratio of 1:10 and 1:1 are, respectively, two- and four-fold lower than in its absence (Figure 2C). This effect is not due to a generic Hsp40 molecular chaperone activity, as it is not observed with Sis1p (Figure 2A), another member of the Hsp40 family. In the presence of an equimolar concentration of Hsp104p, the thioflavin T fluorescence in the elongation phase and at steady state is 2.5-fold higher than in its absence and the lag phase is reduced by a factor of 2 (Figure 2C).
Figure 2.

Assembly of Sup35p in the presence of individual molecular chaperones. (A, B) Time courses of Sup35p (4 μM) assembly at 10°C in the absence of molecular chaperones (•) and in the presence of submolar, 0.4 μM (open symbols), and equimolar (filled symbols) concentrations of Ssa1p (□, ▪), Sis1p (▵, ▴) and Hsp82p (⋄, ♦). (C) Time courses of Sup35p (4 μM) assembly in the absence (•) and in the presence of submolar 0.4 μM (▾, ♦) and equimolar (▴, ▪) concentrations of Ydj1p and Hsp104p, respectively. All assembly reactions were monitored by thioflavin T binding. AU, arbitrary units.
Functional interplay of molecular chaperones during the assembly of Sup35p
To further characterize the influence of molecular chaperones on Sup35p assembly, the following combinations of members from the Hsp40, Hsp70 and Hsp100 families were used: Hsp40/Hsp70, Hsp40/Hsp100, Hsp70/Hsp100 and Hsp40/Hsp70/Hsp100 (Figure 3A–D). Remarkably, in the presence of Ssa1p and Ydj1p or Sis1p (Hsp70/Hsp40 combinations), Sup35p assembly was totally inhibited (Figure 3A). Although we have shown that Ydj1p by itself possesses a significant inhibitory activity, neither Sis1p nor Ssa1p alone display an appreciable ability to inhibit assembly (Figure 2B and C). Thus, Ssa1p acquires an inhibitory capacity only in the presence of its Hsp40 cochaperones. We next studied whether Hsp40 or Hsp70 chaperones influence the stimulatory activity of Hsp104p. When Hsp104p is incubated with Sis1p (Figure 3B), the Sup35p assembly reaction is stimulated to the same extent as in the presence of Hsp104p alone (Figure 2C). In contrast, the assembly reaction of Sup35p in the presence of Hsp104p and Ydj1p (Figure 3B) resembles that obtained in the presence of Ydj1p alone (Figure 2C). This suggests that Ydj1p counteracts the stimulatory activity of Hsp104p, whereas Sis1p has no such effect. Also, the assembly reaction of Sup35p in the presence of Hsp104p and Ssa1p is similar to that in the presence of Hsp104p alone, thus indicating that Ssa1p has a marginal effect on the stimulatory effect of Hsp104p (Figure 3C).
Figure 3.

Functional interplay of molecular chaperones during the assembly of Sup35p. (A–D) Time courses of Sup35p (4 μM) assembly at 10°C in the absence (•) and (A) the presence of equimolar amounts of Ydj1p/Ssa1p (▴) and Sis1p/Ssa1p (□), (B) Ydj1p/Hsp104p (▴) and Sis1p/Hsp104p (♦), (C) Ssa1p/Hsp104p (▴) and (D) Ydj1p/Ssa1p/Hsp104p (▴), Sis1p/Ssa1p/Hsp104p (♦). Assembly was monitored by thioflavin T binding. AU, arbitrary units.
Finally, having demonstrated a total inhibition of Sup35p assembly by the Hsp70–40 system, we examined whether these chaperones might counteract the stimulatory activity of Hsp104p. In a manner similar to what is observed in reactions where Hsp40 family members are associated to Hsp70 in the absence of Hsp104 (Figure 3A), the assembly of Sup35p into protein fibrils is abolished in reactions containing the following combinations Ydj1p/Ssa1p/Hsp104p or Sis1p/Ssa1p/Hsp104p (Figure 3D). This indicates that when combined, Hsp70–40 complexes over-ride the effect of Hsp104p.
The capacity of Ssa1p to inhibit Sup35p fibril formation is greatly stimulated by its cochaperones Sis1p and Ydj1p
The results presented above suggest that Sis1p and Ydj1p enhance the ability of Ssa1p to inhibit Sup35p assembly. To further quantify this effect, Sup35p (4 μM) was assembled in the presence of a constant amount of Ssa1p (4 μM) and increasing concentrations of Ydj1p or Sis1p, and the amount of fibrillar Sup35p at steady state determined using the thioflavin T binding assay. We demonstrate that the addition of submolar amounts of Ydj1p (Figure 4A) or Sis1p (Figure 4B) to Ssa1p causes a dramatic inhibition of Sup35p assembly (50% inhibition is observed in the presence of 0.11 or 0.14 μM Ydj1p and Sis1p, respectively). This inhibition does not correspond to an additive effect of individual molecular chaperones. Sis1p alone has no effect (Figure 4B) and a 10-fold higher concentration of Ydj1p is required to reach comparable assembly inhibition levels (50% inhibition is observed in the presence of 1 μM Ydj1p; Figure 4A). Similar inhibition levels are observed upon lowering the concentration of Ssa1p to 0.5 μM and Sis1p and Ydj1p, respectively, to 0.05 and 0.1 μM (not shown), indicating that Ssa1p acts on Sup35p assembly in a substoichiometric manner.
Figure 4.

Sis1p and Ydj1p stimulate the Sup35p sequestering capacity of Ssa1p. Proportion of fibrillar Sup35p (4 μM) at steady state in the presence of Ssa1p (4 μM) and increasing concentrations (0–8 μM) of (A) Ydj1p (▪) and (B) Sis1p (▪). The thioflavin T fluorescence intensity values at steady state were averaged over three measurements taken at 30, 40 and 50 h following the onset of assembly and expressed as a fraction of the fluorescence of fibrillar Sup35p (4 μM) in the presence of Ssa1p alone. Control reactions where the effect of increasing concentrations of Ydj1p (• in A) and Sis1p (• in B) on the amount of fibrillar Sup35p in the absence of Ssa1p are also presented.
Quantitative analysis of the interplay between Hsp104p and the Hsp70–40 systems on Sup35p assembly
To explore the functional interplay between Hsp104p and Hsp70–40 family members on the assembly of Sup35p into fibrils, we measured the fraction of assembled Sup35p (4 μM) at steady state in the presence of a constant, nonlimiting concentration of Hsp104p (6 μM) and increasing concentrations of Ssa1p and Ydj1p or Sis1p, at an Hsp70:40 ratio of 5:1. This ratio is commonly used in in vitro studies of the disaggregating and folding properties of the Hsp70–40 system (Goloubinoff et al, 1999; Mogk et al, 1999; Krzewska et al, 2001). The fraction of polymerized Sup35p decreases from 100% (arbitrary value) in the presence of Hsp104p alone to about 75% in the presence of a 12-fold excess of Hsp104p over Ssa1p and either Hsp40 cochaperone (Table I). Increased inhibition is observed at lower Hsp104p/Hsp70–40 ratios, and over 85% inhibition takes place using an Hsp104p/Hsp70–40 ratio of 1.5, with equimolar concentrations of Hsp70 and Hsp40 (Table I).
Table 1.
Quantitative analysis of the effect of the interplay between Hsp104p and Hsp70–40 systems on Sup35p assembly
| Concentration of Hsp104p, Hsp70, Hsp40 (μM) | Ratio of Hsp104p/Hsp70–40 | Fraction of assembled Sup35p in the presence of Ssa1p and Ydj1p | Fraction of assembled Sup35p in the presence of Ssa1p and Sis1p |
|---|---|---|---|
| 6, 0, 0 | ∞ | 100 | 100 |
| 6, 4, 0.8 | 1.5 | 41 | 52 |
| 6, 2, 0.4 | 3 | 50 | 59 |
| 6, 1, 0.2 | 6 | 57 | 68 |
| 6, 0.5, 0.1 | 12 | 74 | 77 |
| 6, 4, 4 |
— |
10 |
15 |
| The fraction of assembled Sup35p (4 μM) at steady state in the presence of a constant, nonlimiting concentration of Hsp104p (6 μM) and increasing concentrations of Ssa1p and Ydj1p or Sis1p at an Hsp70/40 ratio of 5:1 are listed. A control measurement at an Hsp70/40 ratio of 1:1 in the presence of Hsp104p (6 μM) is also presented (bottom line). The thioflavin T fluorescence intensity values at steady state were averaged over three measurements made at 30, 40 and 50 h following the onset of assembly and expressed as a fraction of the fluorescence of fibrillar Sup35p (4 μM) in the presence of Hsp104p (6 μM). The results were obtained from two independent measurements. The standard deviation is ±5%. | |||
Ydj1p maintains Sup35p in a soluble state and counteracts the ability of Hsp104p to promote fibril growth
The interaction of Sup35p oligomeric species that form at different stages of assembly with Ydj1p and Hsp104p, separately or together, was further characterized in a quantitative manner using a sedimentation assay. The resulting pellets and supernatants were subjected to SDS–PAGE analysis (Figure 5A). As expected, during the early stage of assembly, in the absence or presence of molecular chaperones, 100% of Sup35p (as determined from densitometric analysis using the software NIH Image) is in the supernatant fractions (Figure 5A). At steady state, in the absence of molecular chaperones, over 85% of Sup35p is in the pellet fraction (Figure 5A). In contrast, Sup35p remains in the supernatants of samples containing Ydj1p alone (Figure 5B) or Ydj1p together with Hsp104p (Figure 5E), whereas 70% of Sup35p is in the pellet fraction in the presence of Hsp104p alone (Figure 5C). These observations are consistent with our kinetic measurements and indicate that Ydj1p maintains Sup35p in a soluble form, thus counteracting Hsp104p activity.
Figure 5.

Effect of Ydj1p and Hsp104p on Sup35p assembly. (A–E and H) Sedimentation analysis of soluble and fibrillar Sup35p (4 μM) (A) in the absence and (B) the presence of Ydj1p (4 μM), (C) Hsp104p (6 μM), (D) Hsp104pTRAP (6 μM) and (E) Ydj1p and Hsp104p (4 and 6 μM, respectively), in the presence of ATP, GTP and an ATP-regenerating system. Aliquots from each sample were subjected to ultracentrifugation at the time indicated. The resulting supernatants (S) and pellets (P) were analyzed by SDS–PAGE. (F, G) Negative-stained electron micrographs of fibrils made from full-length Sup35p (4 μM) in the presence (F) and the absence (G) of Hsp104 (12 μM). Bar, 0.5 μm. Length distribution analysis of 400 fibrils made in the presence and 200 fibrils made in the absence of Hsp104p are presented below the electron micrographs. (H) SDS–PAGE analysis of supernatants (S) and pellets (P) of insoluble fractions of Sup35p fibrils from the second half of the elongation phase in the presence of Hsp104p and AlF4− (S3 and P3), Hsp104p, AlF4− and Ssa1p (S4 and P4), Hsp104p, AlF4− and Ydj1p (S5 and P5) and Hsp104p, AlF4−, Ssa1p and Ydj1p (S6 and P6). Control reactions where Hsp104p alone (S1 and P1) or in the presence of Sup35p fibrils and the absence of AlF4− (S2 and P2) were subjected to the same sedimentation conditions are shown.
Shorter Sup35p fibrils are generated in the presence of Hsp104p, yet no disassembling activity of fibrillar Sup35p is observed
Although Sup35p assembly is stimulated by Hsp104p, we never detect the latter chaperone upon sedimentation of the fibrils, which implies that the interaction, if any, is short-lived or weak. It is possible that the binding capacity of Hsp104p to Sup35p fibrils is lost with time as ATP hydrolysis and nucleotide exchange on Hsp104p following interaction leads to the dissociation of Hsp104p from the fibrils. Thus, we carried out our sedimentation assay using elongating fibrils and either wild-type Hsp104p blocked in its ADP-Pi transition state using the phosphate structural analog, aluminum fluoride (AlF4−), or an Hsp104p mutant, termed Hsp104pTRAP, that binds ATP but is unable to hydrolyze it, because two conserved Glu residues in the Walker B motif of both nucleotide-binding domains have been replaced with Gln (E285Q/E687Q) (Bösl et al, 2005). The ATPase activity of wild-type Hsp104p is markedly reduced (5.5-fold) in the presence of AlF4−, consistent with the binding of AlF4− to Hsp104p in its transition state (Supplementary Figure 1). Nevertheless, the latter form of Hsp104p is not found bound to Sup35p fibrils (Figure 5H). Furthermore, the addition of Ssa1p, Ydj1p or both chaperones to Sup35p fibrils assembled in the presence of Hsp104p and AlF4− has no additional effect, as all chaperones are found in the supernatant (Figure 5H). Similarly, we were not able to reveal a stable interaction between Hsp104p and Sup35p using the variant Hsp104pTRAP (Figure 5D).
Finally, we were unable to detect a stable, physical interaction between soluble, full-length Sup35p and Hsp104p by other classical methods, for example, crosslinking with glutaraldehyde (Supplementary Figure 2) and gel filtration chromatography with both wild-type Hsp104p and the Hsp104TRAP mutant (data not shown).
As examined by electron microscopy, Sup35p fibrils at steady state obtained in the absence and presence of Hsp104p appear morphologically indistinguishable at the resolution used. Strikingly however, in the presence of Hsp104p, the fibrils are significantly shorter than those assembled in its absence (Figure 5F and G, upper panels). This is confirmed by a length distribution analysis (Figure 5F and G, lower panels). In addition, while Sup35p fibrils tend to stack laterally into large bundles, very few form in the presence of Hsp104p (not shown), presumably due to shorter fibrillar length.
Finally, the length of Sup35p fibrils preassembled in the absence of Hsp104p does not change upon further incubation for up to 12 h with Hsp104p (8 μM), as illustrated by the electron micrograph (Supplementary Figure 3). Also, the incubation of Sup35p fibrils, assembled in the absence or presence of Hsp104p for up to 12 h with Hsp104p, Ssa1p and Ydj1p, or Ssa1p and Ydj1p does not lead to fibril depolymerization or solubilization, as the amount of insoluble Sup35p, estimated by our sedimentation assay remains constant (Supplementary Figure 4).
Altogether, this ensemble of data seems to indicate that the full-length Sup35p fibrils that can be sedimented are not a target for Hsp104p activity. Hsp104 neither changes the length of fibrils nor solubilizes them, even with the assistance of Ssa1p and Ydj1p.
Hsp104p acts on Sup35p oligomers that form at the early stages of assembly
To better understand how Hsp104p affects the time course of Sup35p assembly, Hsp104p was added at various times following the onset of the assembly reaction. When Hsp104p is added at time zero, or during the lag phase, this phase shortens and the thioflavin T fluorescence level increases two-fold at steady state (Figure 6); a similar increase is observed upon addition of Hsp104p during the elongation phase (time 20 h). Last, a slow and limited increase in fluorescence is observed when Hsp104p is added at steady state to mature fibrils (time 40 h). However, the fluorescence intensity never reaches the level measured when Hsp104p is added at the earlier stages of assembly. We conclude from these observations that Hsp104p acts on Sup35p oligomers that form at the early stages of assembly.
Figure 6.

Hsp104p promotes the formation of assembly competent Sup35p oligomeric species. Effect of Hsp104p (6 μM) addition on the nucleation (▪), the elongation (▴) and the steady state (♦) phases of Sup35p (4 μM) assembly. The arrows indicate the time where Hsp104p was added to the assembly reaction, time 0, 20 and 40 h. The assembly of Sup35p in the absence of Hsp104p is shown as a control (•). Assembly was carried out at 10°C and monitored using thioflavin T binding. AU, arbitrary units.
Fibrils assembled in the presence of Hsp104p show increased seeding activity
To further characterize the stimulatory effect of Hsp104p on Sup35p assembly, we compared the seeding activities of increasing amounts of Sup35p fibrils preassembled in the absence of Hsp104p to those of a constant amount of fibrils assembled in the presence of increasing amounts of the molecular chaperone.
If Hsp104p promotes Sup35p nucleation, the number of seeds generated in the presence of high Hsp104p concentrations would be higher and therefore have an increased seeding capacity as compared to that obtained in the presence of low Hsp104p concentrations or in its absence, at a constant Sup35p concentration. We find that the apparent rate of Sup35p assembly is accelerated by the addition of increasing amounts of preformed Sup35p fibrils (Figure 7A). As expected, this rate varies linearly with the amount of added seeds in the range 0.5–2 μM (Figure 7B). Importantly, fibrils preassembled in the presence of increasing amounts of Hsp104p (6–18 μM) also show a linear increase in seeding activity, yet significantly higher (Figure 7C), suggesting that more seeds are actually present in the latter samples. When fibrils preassembled in the presence of increasing amounts of Hsp104p are used as seeds, the final concentration of Hsp104p in the elongation reaction is 1.25–3.6 μM. To check whether Hsp104p affects the elongation reaction, the seeding capacities of fibrils assembled without Hsp104p supplemented or not with 3.6 μM of the chaperone were compared. Similar elongation rates are observed in the two reactions (data not shown), thus indicating that the increased nucleation activity of fibrils assembled in the presence of Hsp104p is not due to Hsp104p on its own but to the fibrils.
Figure 7.
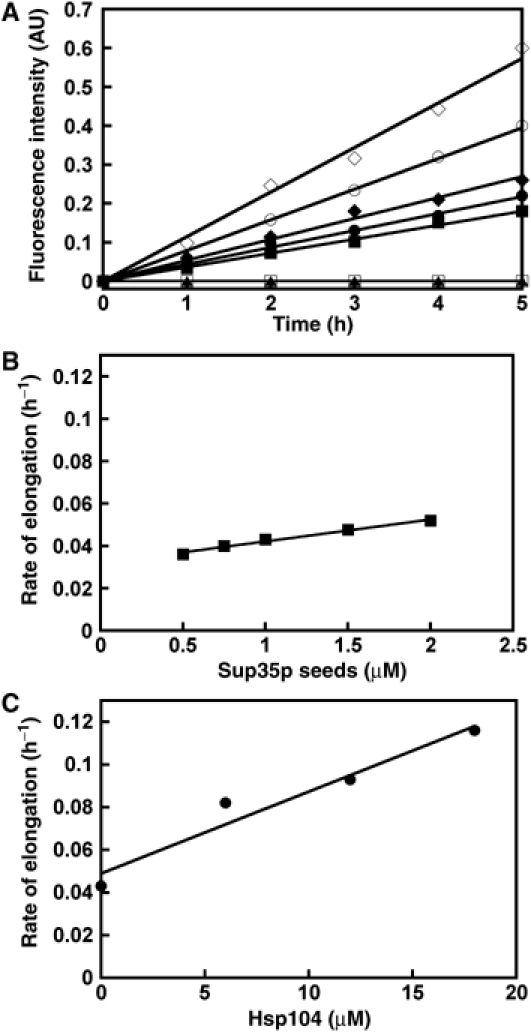
Seeding properties of Sup35p fibrils assembled in the presence and absence of Hsp104p. (A) The elongation rates of increasing concentration of fibrillar Sup35p (0.5, ▪; 1, • and 2 μM, ♦), and that of 1 μM fibrillar Sup35p made in the presence of 6 (○) and 18 μM (⋄) Hsp104p were measured in the presence of 5 μM Sup35p. Control reactions show the assembly of Sup35p in the absence of added seeds (□) and upon addition of 3.6 μM of Hsp104p (▴). The elongation reactions were carried out at 10°C in assembly buffer and were monitored using thioflavin T binding. AU, arbitrary units. (B) Linear dependence between the rate of elongation and the amount of fibrils preformed in the absence of Hsp104p, at a constant soluble Sup35p concentration (5 μM). (C) Linear dependence between the seeding activity of Sup35p fibrils (1 μM) and the amount of Hsp104p present in the reaction where they were assembled. Panels B and C are based on the experiment described in panel A.
Comparison of the elongation rates of seeds made in the presence and the absence of Hsp104p allowed us to estimate the increase in the number of elongation sites due to Hsp104p activity. We show that the apparent assembly rate of 1 μM seeds assembled in the presence of Hsp104p (6–18 μM) is two- to three-fold greater than that of 1 μM seeds assembled in the absence of Hsp104p (Figure 7C). This rate is even greater than that of seeds (2 μM) made in the absence of the chaperone. Altogether, our results strongly suggest a significant increase in the number of elongation sites in the presence of Hsp104p.
Ydj1p binds to oligomeric Sup35p
The oligomeric state of Sup35p in complex with Ydj1p was further studied by size-exclusion chromatography and sedimentation velocity measurements. In the absence of Ydj1p, Sup35p emerges from the column in two peaks (Figure 8A). The first (major peak) elutes just after the column void volume (molecular weight >5 × 105 Da), and the second (minor peak) is centered at 130 kDa. Ydj1p emerges from the same column in a single peak centered at approximately 95 kDa (Figure 8C). When Sup35p is incubated for 2 h in the presence of Ydj1p and the reaction products analyzed on the same column, the amount of Sup35p in the low molecular weight peak decreases and that in oligomeric forms with molecular weights between 130 and 550 kDa increases. In parallel, Ydj1p shifts to higher molecular weight forms, thus suggesting that it binds a range of Sup35p oligomers (Figure 8F).
Figure 8.

Gel filtration analysis of the interaction of molecular chaperones with soluble Sup35p. (A–J) Analysis on 10% SDS–PAGE of protein fractions emerging from a Superose 12 HR column equilibrated and eluted by assembly buffer, (A–I) containing 2 mM ATP, (J) without ATP. (A) Sup35p; (B) Ssa1p; (C) Ydj1p; (D) Sis1p; (E) Sup35p and Ssa1p; (F) Sup35p and Ydj1p; (G) Sup35p and Sis1p; (H) Sup35p, Ssa1p and Ydj1p; and (I, J) Sup35p, Ssa1p and Sis1p. All proteins were loaded at 4 μM. Arrows show the location of molecular size markers (aldolase, 158 kDa; bovine albumin, 66 kDa; ovalbumin, 43 kDa and carboxy anhydrase, 29.5 kDa) run under identical conditions on the same column. Vo (blue dextran, 2000 kDa) is the void volume of the column.
This was further confirmed by analytical ultracentrifugation measurements yielding the apparent distribution of sedimentation coefficients, g*(s), for Sup35p, Ydj1p and Sup35p in the presence of Ydj1p (Supplementary Figure 5). A species with a g*(s) of 108S forms when Sup35p is in the presence of Ydj1p. The other species present in solution have sedimentation coefficients of 55 and 9.3S representing Sup35p and Ydj1p, respectively. The soluble Sup35p–Ydj1p complex is therefore two- to three-fold larger than soluble Sup35p oligomers.
Ssa1p binds to oligomeric Sup35p species only in the presence of Hsp40
One possible scenario that accounts for the action of the Hsp70–40 system is that Ssa1p in the presence of its cochaperones inhibits Sup35p assembly by sequestering Sup35p oligomeric species that act as precursors of the fibrillar form. To test this possibility, we performed size-exclusion chromatography experiments with Sup35p and Ssa1p in the presence of Ydj1p or Sis1p (4 μM each). In accord with our kinetic data, no evidence for a Sup35p–Ssa1p complex is observed upon incubation of the two proteins, since they elute from the column in fractions compatible with their respective molecular weight (compare Figure 8E with 8A and B). Significant changes in the elution profiles are observed upon addition of Ydj1p or Sis1p to a Sup35p/Ssa1p mixture. A shift is seen, whereby Ssa1p coelutes from the column with Sup35p and Ydj1p or Sis1p (Figure 8H and I, respectively), strongly suggesting the formation of the ternary complexes, Sup35p–Ssa1p–Sis1p and Sup35p–Ssa1p–Ydj1p. The formation of these complexes is ATP dependent as no such complexes are observed without ATP as demonstrated using the reaction containing Sup35p, Ssa1p and Sis1p (Figure 8J).
Discussion
The faithful propagation of [PSI+] in yeast is a complex process highly dependent on the expression of molecular chaperones (Tuite and Cox, 2003). While the expression levels of a number of molecular chaperones, exemplified by Hsp82p, do not affect [PSI+] propagation (Newnam et al, 1999), other chaperones are strictly required and play a crucial role in the propagation of the prion. Hsp104p best illustrates this group of molecular chaperones, as yeast strains carrying an HSP104 gene deletion are unable to propagate [PSI+] (Chernoff et al, 1995). Members of the Hsp40 and Hsp70 chaperone families also alter [PSI+] propagation, although they have complex effects and are [PSI+] variant specific (Chernoff et al, 1999; Newnam et al, 1999; Kushnirov et al, 2000; Jones and Masison 2003; Allen et al, 2005). A transient increase in the expression level of Ssa1p has been reported to stimulate [PSI+] in different variants (Newnam et al, 1999), whereas it antagonizes [PSI+] in other atypical strains (Kushnirov et al, 2000; Borchsenius et al, 2001). In all cases, the integrity of Ssa1p, that is, its ATPase activity and its capacity to interact with an Hsp40 cochaperone are critical for its role in [PSI+] propagation (Jung et al, 2000; Jones and Masison, 2003; Jones et al, 2004).
So far, the roles of molecular chaperones have been essentially documented using in vivo approaches. Herein, we describe the effects of molecular chaperones, individually and in combination, on the assembly of the full-length Sup35p in vitro. We show that Hsp104p greatly stimulates the assembly of Sup35p into fibrils, whereas Ydj1p inhibits it. Hsp82p has no effect on assembly, a result supported by in vivo studies (Newnam et al, 1999). Similarly, neither Ssa1p nor Sis1p individually influence Sup35p assembly. Remarkably however, when Ssa1p is coupled to either of its Hsp40 cochaperones (Ydj1p or Sis1p), the assembly of Sup35p is strongly inhibited.
Here we show that Hsp104p reduces the nucleation phase preceding assembly and increases the apparent assembly rate of the full-length Sup35p. This is in agreement with the effect of Hsp104p on Sup35pNM fragment nucleation, as reported previously (Shorter and Lindquist, 2004).
Having demonstrated a very strong stimulatory effect of Hsp104p on Sup35p assembly, we discovered that the chaperone neither changes the length of preformed fibrils nor solubilizes them, even with the assistance of Ssa1p and Ydj1p. This tends to indicate that, at least under our experimental conditions, fibrillar Sup35p, that sediments upon ultracentrifugation, is not a suitable substrate for Hsp104p. Interestingly, Hsp104p has been reported to disassemble fibrils composed of Sup35pNM fragment in vitro (Shorter and Lindquist, 2004). One possible reason for the discrepancy between these results may come from the molecular crowding due to the C-terminal region of Sup35p, representing nearly two-thirds of the protein, in fibrils made of the full-length Sup35p. Indeed, fibrils of the full-length Sup35p and its NM fragment possess different quaternary structure (Glover et al, 1997). It is also reasonable to envisage that polymers made of the full-length Sup35p and Sup35pNM expose different surfaces area to the solvent. Finally, Hsp104p has been reported to bind Sup35pNM fibrils labelled with a hydrophobic, fluorescent dye, and exhibits a severing activity only upon addition of cell extracts (Inoue et al, 2004). Thus, the absence of a severing activity using purified full-length Sup35p and Hsp104p do not rule out the possibility that yet unidentified cellular components are involved in or possess such an activity.
Hsp104p shortens the lag phase preceding assembly. This implies that it must interact with soluble oligomeric species of Sup35p that form in the early stages of assembly. Evidences that Hsp104p modulates the oligomeric state of Sup35p oligomers have been reported (Schirmer and Lindquist, 1997; Kryndushkin et al, 2003).
The interaction of Hsp104p with soluble Sup35p oligomers stimulates nucleation and elongation. Different scenarios can be envisaged to account for this observation. By interacting with oligomeric species of Sup35p that are destined to assemble into fibrils, Hsp104p would displace the equilibrium between different oligomeric forms of Sup35p toward the oligomeric form that constitutes the precursor of fibrillar Sup35p. Alternatively, the interaction between Hsp104p and soluble oligomeric Sup35p might prevent or reverse oligomerization pathways leading towards amorphous aggregation. As a consequence, a higher proportion of assembly-competent Sup35p oligomers may form in the presence of Hsp104p, thus leading to improved assembly, for example, a shorter lag phase and an increased number of short fibrils, with a low propensity to assemble laterally into bundles, in contrast with the long fibrils generated in the absence of Hsp104p. If this is indeed what occurs in the cytoplasm, our in vitro observations might be accounting for the involvement of Hsp104p in the faithful propagation of [PSI+]. Indeed, the short Sup35p polymers generated in the presence of Hsp104p will segregate with greater efficiency between mother and daughter cells than the larger aggregates that form in the absence or upon inactivation of the molecular chaperone.
Despite our kinetic evidence indicating that soluble oligomeric Sup35p is the target of Hsp104p activity, we were unable to detect a physical interaction between soluble Sup35p and Hsp104p by glutaraldehyde crosslinking and gel filtration chromatography experiments, even when Hsp104p was blocked in its high-affinity ATP-like state. This is in accord with the findings made by others when they attempted to demonstrate a physical interaction between Sup35p and Hsp104p (Schirmer and Lindquist, 1997; Allen et al, 2005).
In contrast to Hsp104p, we show that Ydj1p significantly blocks the assembly of Sup35p into fibrils. Ydj1p stably interacts with a range of oligomeric Sup35p species and the complexes can be observed in size-exclusion chromatography and sedimentation velocity experiments. This interaction maintains Sup35p in a soluble, assembly-incompetent state, as confirmed by our sedimentation assay and the kinetic measurements. However, this is not a generic property of Hsp40 family members as Sis1p neither binds to Sup35p oligomers nor affects significantly Sup35p assembly. Ssa1p does not interact on its own with soluble Sup35p nor affect its assembly. It is only when Ssa1p cochaperones Sis1p and Ydj1p are present that a physical interaction between Ssa1p and Sup35p oligomers is observed. The formation of Sup35p–Ssa1p–Sis1p/Ydj1p ternary complexes is ATP dependent and yields assembly-incompetent Sup35p. Thus, Ssa1p acquires a Sup35p-sequestering activity when partnered to either of its Hsp40 cochaperones. The latter activity of Ssa1p is probably due to the stimulation of the Ssa1p ATPase activity to a similar extent by Sis1p or Ydj1p, as proposed previously (Fan et al, 2004). It is interesting to note that an equivalent suppressive effect has been reported for the Hsp70–40 chaperone pair on the assembly of a fragment of huntingtin-containing expanded polyglutamine tracts (Muchowski et al, 2000).
Under optimal growth conditions where [PSI+] is faithfully propagated, the basal expression level of Hsp104p is increased (Jung et al, 2000). When Hsp104p, Ssa1p and Ydj1p are overexpressed individually, a curative effect that depends on the [PSI+] variant is observed (Chernoff et al, 1995; Kushnirov et al, 2000). However, the [PSI+]-curing effect induced by the overexpression of Hsp104p is partially counteracted by the coexpression of Ssa1p (Newnam et al, 1999; Allen et al, 2005). Interestingly, when the expression levels of all HSPs are elevated, for example, under natural stress conditions exemplified by heat shock at 37–39°C or at 52°C, the mitotic stability of [PSI+] was marginally or not affected (Tuite, 1978; Tuite et al, 1981; Cox et al, 1988). These observations have nurtured the view that high levels of Ssa1p could play a putative protective role in [PSI+] maintenance when Hsp104p expression is also elevated, for example, under stress conditions or during the stationary phase (Newnam et al, 1999; Serio and Lindquist, 2000). The ensemble of these observations suggests that [PSI+] propagation is under the strong influence of molecular chaperones' expression levels, and also that the observed effects depend as much on their individual as on their relative concentrations.
Our study reveals that the pronounced stimulatory effect of Hsp104p on Sup35p assembly can be partially over-ruled by the inhibitory effect of Ydj1p on its own and to a greater extent by Ssa1p when associated to Ydj1p or Sis1p. This rather implies that Ssa1p, assisted by its Hsp40 cochaperone, sequesters a range of Sup35p species thus counteracting Hsp104p action. To better understand the interplay between molecular chaperones on Sup35p assembly, we examined the effect of Hsp104p in the presence of increasing ratios of the Hsp70–40 chaperone system. Remarkably, the stimulatory effect of Hsp104p is substantially counteracted by submolar amounts of Ssa1p–Ydj1p and Ssa1p–Sis1p. It is only when the concentration of the Hsp70–40 system is over an order of magnitude lower than that of Hsp104p that the stimulatory effect of the latter on Sup35p assembly is only partially affected. The combined effects of Ssa1p and Ydj1p or Sis1p could provide a plausible explanation for the modulation of Hsp104p activity in the propagation of [PSI+] through changes in their cellular levels.
Overall, our study reveals the functional interplay between Hsp104p and the Hsp70–40 systems in the assembly of Sup35p into protein fibrils. This interplay might account for a precise modulation of Hsp104p activity in the propagation of [PSI+] through changes in the cellular levels of Ssa1p, Ydj1p and Sis1p molecular chaperones. Indeed, Ssa1p in combination with its Hsp40 cochaperones may bind to a range of Sup35p oligomeric species with higher affinity than Hsp104p, thus sequestering Sup35p in an assembly-incompetent state and disfavoring further oligomerization and assembly into fibrils. Alternatively, the Hsp70–40 family members may interact specifically with Sup35p oligomeric species that are generated in the presence of Hsp104p, for example, at their growing ends, thus annihilating the effect of Hsp104p and preventing the continued polymerization of Sup35p assembly-competent forms.
A number of models have been proposed to account for the roles played by molecular chaperones in the maintenance and destabilization of prion propagation (reviewed in Tuite and Cox, 2003). One model hypothesizes that the elevated levels of molecular chaperones facilitate the disaggregation of the high molecular weight species of prion proteins that can act as seeds. Our data bring no evidence for such scenario, although as discussed, this may be due to the limits of an in vitro system lacking important yet unidentified cellular factors crucial for such a scenario to occur. Another model proposes that the faithful transmission of the high molecular weight species of prion proteins from mother to daughter cells, is compromised upon the overexpression of molecular chaperones. The assembly-promoting activity of Hsp104p discussed here is certainly consistent with this model in particular when it is not counteracted by Hsp70–40 action. In one last model, the molecular chaperones sequester either the folding intermediate(s) that assemble into prion aggregates or the cellular factor(s) that are required for the generation of the high molecular weight species of prion proteins that act as seeds. The inhibitory effects of Ydj1p and of Ssa1p in conjunction with its Hsp40 cochaperones exemplify such a model. As mentioned above, additional scenarios such as capping one or the two ends of growing fibrils, or interacting with fibril walls and allowing the bundling or the disordered aggregation of the fibrils into very large aggregates, can account for the assembly inhibitory effect of a number of molecular chaperones.
The individual steps in the in vitro assembly reaction of soluble Sup35p that are modulated by the activity of the molecular chaperones used in our in vitro study are represented in Figure 9. Further identification of Sup35p partner proteins, for example, by a proteomic approach, and subsequent characterization of their roles, should allow a better understanding of the prion conversion pathways and lead to a complete functional scheme for the assembly of Sup35p into prion particles.
Figure 9.

Schematic representation of the assembly of Sup35p into insoluble high molecular weight oligomers and the effect of the different molecular chaperones used on the assembly reaction. Soluble assembly-competent Sup35p form presumably through a reversible unfolding event, which extent is unknown. This assembly-competent form oligomerizes into soluble high molecular weight oligomeric forms that can either grow by incorporation of soluble assembly-competent Sup35p or by condensation yielding insoluble high molecular weight forms, for example, fibrils. Hsp104p promotes the formation of the soluble high molecular weight oligomers that act as nuclei in Sup35p assembly. Ydj1p binds either soluble Sup35p folding intermediates or assembly-competent Sup35p, thus forming a Sup35p–Ydj1p assembly-incompetent binary complex. Ssa1p on its own, in the presence or the absence of ATP, has no significant influence on the assembly pathway. It is only in the presence of its Hsp40 cochaperone and ATP that an assembly-incompetent ternary complex (Sup35p–Ssa1p–Ydj1p or Sis1p) forms. The width of each arrow depicts the strength with which the assembly pathway is displaced from its normal fate.
Materials and methods
Construction of expression vectors
The open-reading frame of the SUP35 gene was amplified using the following oligonucleotide primers: 5′-GGGAAGCAATAATTCATATGTCGGATTCAAAC CAAGGC-3′ and 5′-TTACGCGGATCCTTACTCGGCAATTTTAACAA TTTTACCC-3′.
For cloning of the SIS1 gene, the oligonucleotide primers,5′-GGAATTCATATGGTCAAGGAGACAAAAC-3′ and 5′-CGCGGATCCTTAAAAATTTTCATCTATAGC-3′, were used. Both amplification products were cloned into the pET15b vector, in the NdeI and BamHI sites.
pET15b-YDJ1 and pET15b-HSP104 were kindly provided by Dr S Burston and Dr D Poso (University of Bristol, UK). The yeast expression vector p416 TEF His SSA1 was a kind gift of Professor E Craig (University of Wisconsin, Madison, WI).
Expression and purification of recombinant proteins
Sup35p, Hsp104p, Ydj1p and Sis1p were overexpressed in Escherichia coli strain BL21-CodonPlus, in 2 × YT media complemented with chloramphenicol (34 μg/ml) and carbenicillin (100 μg/ml), at 30°C. Protein expression was induced with 1 mM IPTG, at OD600=0.5–0.7. The bacterial pellets were resuspended in buffer A (20 mM Tris–HCl, pH 8.0, 1 M NaCl, 20 mM imidazole, 5 mM β-mercaptoethanol, 5% glycerol) for Sup35p, in buffer B (20 mM Tris–HCl, pH 8.0, 0.5 M NaCl, 20 mM imidazole, 0.01% Triton X-100, 5 mM β-mercaptoethanol, 10% glycerol) for Hsp104p and in buffer C (20 mM Tris–HCl, pH 8.0, 0.5 M NaCl, 20 mM imidazole, 10% glycerol) for Ydj1p and Sis1p, supplemented with EDTA-free inhibitor cocktail tablets (Complete, Roche Diagnostics Gmbh, Mannheim, Germany). The cells were disrupted by sonication. The lysates were clarified by centrifugation and loaded directly onto the Chelating Sepharose column (Amersham Biosciences) equilibrated in the respective lysis buffers. Bound proteins were eluted with linear gradients of imidazole (20–500 mM). Peak fractions containing Sup35p, Hsp104p, Ydj1p and Sis1p were dialysed against buffer D (20 mM Tris–HCl, pH 8.0, 1 M NaCl, 5 mM β-mercaptoethanol, 5% glycerol), buffer E (50 mM Tris–HCl, pH 8.0, 200 mM NaCl, 5 mM β-mercaptoethanol, 0.01% Triton X-100, 5% glycerol), buffer F (20 mM Tris–HCl, pH 7.5, 50 mM NaCl, 5 mM β-mercaptoethanol, 10% glycerol) and buffer G (50 mM Tris–HCl, pH 7.5, 150 mM KCl, 5 mM β-mercaptoethanol, 10% glycerol), respectively, and stored at −80°C. For successful full-length Sup35p purification, it is crucial that bacterial harvesting, preparation of the extract and the single purification step are performed subsequently at 8°C and in a time frame not exceeding 6 h. In the case of Ydj1p, an additional purification step was required prior to storage. Ydj1p was loaded onto the QFF Sepharose column (Amersham Biosciences). The column was washed with buffer F supplemented with 150 mM NaCl, and the proteins were eluted with a linear gradient of NaCl (150–500 mM). Fractions containing Ydj1p were dialyzed against buffer H (50 mM Tris–HCl, pH 7.5, 100 mM NaCl, 5 mM DTT, 10% glycerol), concentrated (VivaSpin 30 000 concentrators) and stored at −80°C.
Ssa1p was overexpressed in S. cerevisiae strain W3031b/p416 TEF His SSA1. Cells were grown at 30°C in SSC medium (uracil deficient), complemented with 2% glucose. They were harvested at OD600=2. The cell pellet was resuspended in buffer C, supplemented with EDTA-free inhibitor cocktail tablets and disrupted using a French Press. The lysate was clarified by centrifugation and loaded directly onto a HiTrap Chelating HP column (Amersham Biosciences). The following steps in the purification of Ssa1p are identical to those of Ydj1p with the exception that NaCl was replaced by KCl.
Purified Hsp82p (Prodromou et al, 1996) was generously provided by Dr C Combeau (Sanofi-Aventis Pharma, Vitry, France). Purified Hsp104E285Q/E687Q variant was a kind gift of Dr S Walter (Technische Universitat, Munchen, Germany).
Assembly of Sup35 into protein fibrils
Sup35p and the molecular chaperones were dialyzed against the assembly buffer (20 mM Tris–HCl, pH 8.0, 200 mM NaCl, 5% glycerol, 5 mM β-mercaptoethanol, 10 mM MgCl2). After dialysis, Sup35p was diluted into the assembly buffer containing various combinations of molecular chaperones and 5 mM ATP, 5 mM GTP and an ATP-regenerating system (10 mM creatine phosphate, 100 μg/ml creatine kinase). The reaction mixtures were incubated at 10°C. At regular time intervals, aliquots were removed and the assembly reaction was monitored using a thioflavin T binding assay (McParland et al, 2000). Thioflavin T binding was measured by averaging the emission signal over 60 s using a Quantamaster QM 2000-4 spectrofluorometer (Photon Technology International, NJ).
Sedimentation assay
Soluble Sup35p at the concentration indicated in the text (usually 4–5 μM) was assembled into fibrils in the presence of the different molecular chaperones, as described above. At the times indicated in the text, aliquots (50 μl) were removed from each solution and subjected to ultracentrifugation (27 000 g at 10°C for 20 min) in a TL100 tabletop Beckman Ultracentrifuge. Pellets were dried out in a SpeedVac apparatus (Millipore), then 5 μl of 7 M urea solution was added to each tube and the sample incubated for 15 min, with agitation, at 37°C. Fractions of supernatants and pellets were denatured and analyzed using SDS–PAGE (Laemmli, 1970).
Electron microscopy
Samples of fibrillar Sup35p made in the presence and the absence of Hsp104p were negatively stained on carbon-coated grids (200 mesh) with 1% uranyl acetate and examined in a Philips EM 410 electron microscope.
Sedimentation velocity
Sedimentation velocity experiments were carried out on a Beckman Optima XL-A analytical ultracentrifuge, equipped with a 60 Ti four-hole rotor and cells with two-channel 12-mm path length centerpieces. Samples were centrifuged at 20 000 and 40 000 r.p.m. at 10°C. Radial scans of absorbance at 280 nm were taken at 6-min intervals. Data analysis to provide the apparent distribution of sedimentation coefficients was performed using the program SVEDBERG (Philo, 1994).
Size-exclusion chromatography
Sup35p, Ssa1p, Sis1p and Ydj1p were dialyzed against the assembly buffer. After dialysis, Sup35p was subjected to ultracentrifugation (27 000 g at 8°C for 20 min) in a TL100 tabletop Beckman Ultracentrifuge. Sup35p, Ydj1p, Ssa1p and Sis1p alone and in various combinations were incubated in the assembly buffer supplemented with 2 mM ATP, unless specified. The mixtures were loaded onto Superose 12 HR 10/30 column (Amersham Biosciences) equilibrated in assembly buffer containing 2 mM ATP, unless specified. Chromatography was carried out at a flow rate 0.6 ml/min at 8°C. Fractions (600 μl) were collected and analyzed by SDS–PAGE.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Acknowledgments
We gratefully acknowledge Dr Philip Simister for helpful discussions, thorough editing of the manuscript and his help in gel filtration experiments. We thank Dr Steven Burston and Professor Antony Clarke for allowing the initiation of this project and Dr Fatima El Khadali for the help with analytical ultracentrifugation measurements. We are grateful to Dr Stefan Walter for purified Hsp104pTRAP mutant, Dr Cecile Combeau for purified Hsp82p and to Professor Elisabeth Craig, Dr Steven Burston and Dr Daniel Poso for kindly providing plasmids. This work was supported by the French Ministry of Education, Research and Technology through the Groupement d'Intérêt Scientifique Prion; the Centre National de la Recherche Scientifique (CNRS); and by the Fondation Recherche Médicale (FRM).
References
- Allen KD, Wegrzyn RD, Chernova TA, Muller S, Newnam GP, Winslett PA, Wittich KB, Wilkinson KD, Chernoff YO (2005) Hsp70 chaperones as modulators of prion life cycle: novel effects of Ssa and Ssb on the Saccharomyces cerevisiae prion [PSI+]. Genetics 169: 1227–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchsenius AS, Wegrzyn RD, Newnam GP, Inge-Vechtomov SG, Chernoff YO (2001) Yeast prion protein derivative defective in aggregate shearing and production of new ‘seeds'. EMBO J 20: 6683–6691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bösl B, Grimminger V, Walter S (2005) Substrate binding to the molecular chaperone Hsp104 and its regulation by nucleotides. J Biol Chem 280: 33170–38176 [DOI] [PubMed] [Google Scholar]
- Chernoff YO, Lindquist SL, Ono B, Inge-Vechmotov SG, Liebman SW (1995) Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+]. Science 268: 880–884 [DOI] [PubMed] [Google Scholar]
- Chernoff YO, Newnam GP, Kumar J, Allen K, Zink AD (1999) Evidence for a protein mutator in yeast: role of the Hsp70 related chaperone ssb in formation, stability, and toxicity of the [PSI+] prion. Mol Cell Biol 19: 8103–8112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BS (1965) PSI, a cytoplasmic suppressor of super-suppressor in yeast. Heredity 20: 505–521 [Google Scholar]
- Cox BS, Tuite MF, McLaughlin CS (1988) The psi factor of yeast: a problem in inheritance. Yeast 4: 159–178 [DOI] [PubMed] [Google Scholar]
- Derkatch IL, Chernoff YO, Kushnirov VV, Inge-Vechtomov SG, Liebman SW (1996) Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics 144: 1375–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CY, Lee S, Ren HY, Cyr DM (2004) Exchangeable chaperone modules contribute to specification of type I and type II Hsp40 cellular function. Mol Biol Cell 15: 761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover JR, Kowal AS, Schirmer EC, Patino MM, Liu JJ, Lindquist S (1997) Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell 89: 811–919 [DOI] [PubMed] [Google Scholar]
- Glover JR, Lindquist S (1998) Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94: 73–82 [DOI] [PubMed] [Google Scholar]
- Goloubinoff P, Mogk A, Zvi AP, Tomoyasu T, Bukau B (1999) Sequential mechanism of solubilization and refolding of stable protein aggregates by a bichaperone network. Proc Natl Acad Sci USA 96: 13732–13737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Taguchi H, Kishimoto A, Yoshida M (2004) Hsp104 binds to yeast Sup35 prion fiber but needs other factor(s) to sever it. J Biol Chem 279: 52319–52323 [DOI] [PubMed] [Google Scholar]
- Jones G, Song Y, Chung S, Masison D (2004) Propagation of Saccharomyces cerevisiae [PSI+] prion is impaired by factors that regulate Hsp70 substrate binding. Mol Cell Biol 24: 3928–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GW, Masison DC (2003) Saccharomyces cerevisiae Hsp70 mutations affect [PSI+] prion propagation and cell growth differently and implicate Hsp40 and tetratricopeptide repeat cochaperones in impairment of [PSI+]. Genetics 163: 495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G, Jones G, Wegrzyn RD, Masison DC (2000) A role for cytosolic Hsp70 in yeast [PSI+] prion propagation and [PSI+] as a cellular stress. Genetics 156: 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CY, Diaz-Avalos R (2004) Protein-only transmission of three yeast prion strains. Nature 428: 319–323 [DOI] [PubMed] [Google Scholar]
- King CY, Tittmann P, Gross H, Gebert R, Aebi M, Wuthrich K (1997) Prion-inducing domain 2–114 of yeast Sup35 protein transforms in vitro into amyloid-like filaments. Proc Natl Acad Sci USA 94: 6618–6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryndushkin S, Alexandrov I, Ter-Avanesyan MD, Kushnirov VV (2003) Yeast [PSI+] prion aggregates are formed by small Sup35 polymers fragmented by Hsp104. J Biol Chem 278: 49636–49643 [DOI] [PubMed] [Google Scholar]
- Krzewska J, Langer T, Liberek K (2001) Mitochondrial Hsp78, a member of the Clp/Hsp100 family in Saccharomyces cerevisiae, cooperates with Hsp70 in protein refolding. FEBS Lett 489: 92–96 [DOI] [PubMed] [Google Scholar]
- Kushnirov VV, Kryndushkin DS, Boguta M, Smirnov VN, Ter-Avanesyan MD (2000) Chaperones that cure yeast artificial [PSI+] and their prion-specific effects. Curr Biol 10: 1443–1446 [DOI] [PubMed] [Google Scholar]
- Kushnirov VV, Ter-Avanesyan MD (1998) Structure and replication of yeast prions. Cell 94: 13–16 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 27: 680–685 [DOI] [PubMed] [Google Scholar]
- McParland VJ, Kad NM, Kalverda AP, Brown A, Kirwin-Jones P, Hunter MG, Sunde M, Radford SE (2000) Partially unfolded states of β2-microglobulin and amyloid formation in vitro. Biochemistry 39: 8735–8746 [DOI] [PubMed] [Google Scholar]
- Mogk A, Tomoyasu T, Goloubinoff P, Rudiger S, Roder D, Langen H, Bukau B (1999) Identification of thermolabile Escherichia coli proteins: prevention and reversion of aggregation by DnaK and ClpB. EMBO J 18: 6934–6949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski PJ, Schaffer G, Sitter A, Wanker EE, Hayer-Hartl MT, Hartl U (2000) Hsp70 and Hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc Natl Acad Sci USA 97: 7841–7846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newnam GP, Wegrzyn RD, Lindquist SL, Chernoff YO (1999) Antagonistic interactions between yeast chaperones Hsp104 and Hsp70 in prion curing. Mol Cell Biol 19: 1325–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell DA, Kowal AS, Singer MA, Lindquist S (1994) Protein disaggregation mediated by heat-shock protein Hsp104. Nature 372: 475–478 [DOI] [PubMed] [Google Scholar]
- Patino MM, Liu JJ, Glover JR, Lindquist S (1996) Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science 273: 622–626 [DOI] [PubMed] [Google Scholar]
- Paushkin SV, Kushnirov VV, Smirnov VN, Ter-Avanesyan MD (1996) Propagation of the yeast prion-like [PSI+] determinant is mediated by oligomerisation of the SUP35-encoded polypeptide chain release factor. EMBO J 15: 3127–3134 [PMC free article] [PubMed] [Google Scholar]
- Philo JS (1994) Measuring sedimentation, diffusion, and molecular weights of small molecules by direct fitting of sedimentation velocity concentration profiles. In Modern Analitical Ultracentrifugation, Schuster TM, Laue TM (eds), pp 156–170. Boston: Birkhauser [Google Scholar]
- Prodromou C, Piper PW, Pearl LH (1996) Expression and crystallisation of the yeast Hsp82 chaperone, and preliminary X-ray diffraction studies of the amino-terminal domain. Proteins Struct Funct Genet 25: 517–522 [DOI] [PubMed] [Google Scholar]
- Serio TR, Cashikar AG, Kowal AS, Sawicki GJ, Moslehi JJ, Serpell L, Arnsdorf MF, Lindquist SL (2000) Nucleated conformational conversion and the replication of conformational information by a prion determinant. Science 289: 1317–1321 [DOI] [PubMed] [Google Scholar]
- Serio TR, Lindquist SL (2000) Protein-only inheritance in yeast: something to get [PSI+]-ched about. Trends Cell Biol 10: 98–105 [DOI] [PubMed] [Google Scholar]
- Schirmer EC, Lindquist S (1997) Interactions of the chaperone Hsp104 with yeast Sup35 and mammalian PrP. Proc Natl Acad Sci USA 94: 13932–13937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J, Lindquist S (2004) Hsp104 catalyzes formation and elimination of self-replicating Sup35 prion conformers. Science 304: 1793–1797 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Chien P, Naber N, Cooke R, Weissman JS (2004) Conformational variations in an infectious protein determine prion strain differences. Nature 428: 323–328 [DOI] [PubMed] [Google Scholar]
- Tuite MF (1978) Genetics of nonsense suppressors in yeast. D Phil Thesis, University of Oxford
- Tuite MF, Cox BS (2003) Propagation of yeast prions. Nat Rev Mol Cell Biol 4: 878–890 [DOI] [PubMed] [Google Scholar]
- Tuite MF, Mundy CR, Cox BS (1981) Agents that cause a high frequency of genetic change from [psi+] to [psi−] in Saccharomyces cerevisiae. Genetics 98: 691–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegrzyn RD, Bapat K, Newnam GP, Zink AD, Chernoff YO (2001) Mechanism of prion loss after Hsp104 inactivation in yeast. Mol Cell Biol 21: 4656–4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB (1994) Evidence for a prion analog in S. cerevisiae: the [URE3] non-Mendelian genetic element as an altered URE2 protein. Science 264: 566–569 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5


