Abstract
The selective reversible S-glutathiolation of specific SERCA (sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase) cysteine residues represents a novel physiologic pathway of NO (nitric oxide)-dependent arterial smooth muscle relaxation [Adachi, Weisbrod, Pimentel, Ying, Sharov, Schöneich and Cohen (2004) Nat. Med. 10, 1200–1207]. This mechanism may be impaired through the irreversible oxidation of functionally important cysteine residues as a consequence of oxidative stress and aging. To establish whether in vivo aging and in vitro oxidation by peroxynitrite result in the loss of such functionally important cysteine residues of SERCA, we have developed and optimized a quantitative method to monitor the oxidation state of the individual SERCA cysteine residues using a maleimide-based fluorescence dye, TG1 (ThioGlo® 1), as a label for cysteine residues that have not been altered by oxidation and are not involved in disulphide bridges. A high efficiency for TG1 labelling of such residues and the chemical structure of cysteine–TG1 adducts were validated by MS analysis of model peptides, model proteins and rat skeletal muscle SERCA1. Tryptic peptides containing 18 out of a total of 24 cysteine residues were identified by HPLC–ESI (electrospray ionization)–MS/MS (tandem MS). Two cysteine residues, at positions 344 and 349, were detected in the form of an internal disulphide bridge, and another 16 were found to be labelled with TG1. Using HPLC–ESI–MS, we quantitatively mapped peroxynitrite oxidation of eight cysteine residues (positions 364, 417, 420, 498, 525, 674, 675 and 938), some of which are involved in the control of SERCA activity. Biological aging resulted in the partial modification of cysteine residues 377, 498, 525, 561, 614, 636, 674, 675, 774 and 938. Neither peroxynitrite exposure nor biological aging affected the apparent SERCA1 ATP affinity. Our data show an age-dependent loss of cysteine residues (approx. 2.8 mol of cysteine/mol of SERCA1), which may be partially responsible for the age-dependent decrease in the specific Ca2+-ATPase activity (by 40%).
Keywords: aging, Ca2+-ATPase, cysteine oxidation, quantitative mapping, sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase (SERCA), sarcoplasmic reticulum
Abbreviations: a.m.u., atomic mass units; CaM, calmodulin; DTT, dithiothreitol; ER, endoplasmic reticulum; ESI, electrospray ionization; MS/MS, tandem MS; NAC, N-acetyl-L-cysteine; 3-NT, 3-nitrotyrosine; RP-HPLC, reversed-phase HPLC; SERCA, sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase; SR, sarcoplasmic reticulum; TG1, ThioGlo® 1; 4-VP, 4-vinylpyridine
INTRODUCTION
SERCA (sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase) plays a key role in the relaxation of smooth, cardiac and skeletal muscle through the transport of cytosolic Ca2+ into the SR (sarcoplasmic reticulum) or ER (endoplasmic reticulum) [1,2]. However, other physiological and also pathological processes are associated with an abnormal activity or expression of SERCA, such as development, cell proliferation [3–5], apoptosis [6,7], Brody and Darier disease, and cancer [8–10]. Three genes coding for SERCA proteins have been identified (SERCA1, SERCA2 and SERCA3), but the mRNA in each case can undergo differential processing to produce various isoforms. For example, recent studies have identified six isoforms of SERCA3 alone, designated SERCA3a–SERCA3f [11,12]. Expression of SERCA1 is restricted to fast-twitch skeletal muscle, while SERCA2a is only produced in cardiac and slow-twitch skeletal muscle [2]. In contrast, SERCA2b and SERCA3 are present in a large variety of tissues. An important feature of SERCA is its high sensitivity towards modification by reactive oxygen species. In arteries, physiological levels of NO (nitric oxide) cause site-specific S-glutathiolation of Cys669 and/or Cys674, associated with approx. 50% increase in activity, indicated through a series of pharmacological and mass spectrometric experiments [13]. This novel mechanism is associated with NO-dependent vascular relaxation. Importantly, this effect requires the simultaneous formation of NO and superoxide (O2−), suggesting the intermediacy of peroxynitrite (ONOO−). In contrast, higher, more pathological, levels of NO or peroxynitrite cause inactivation of the enzyme [14]. Such inactivation of SERCA can be physiologically detrimental, as a reduced function of SERCA has been associated with a changed cellular response to apoptotic stimuli, and also with an increased risk of cancer [7].
Our in vivo studies have documented that biological aging leads to oxidation and nitration of SERCA, at cysteine and tyrosine residues respectively, accompanied by partial inactivation of the protein. Predominantly for SERCA2a from aged slow-twitch skeletal muscle [15] and heart [16], MS allowed us to identify the accumulation of 3-NT (3-nitrotyrosine) at positions 294 and 295, which are located at the lumen–membrane interface of the transmembrane helix M4, and at position 753. Quantitative analysis indicated the age-dependent accumulation of up to 4 mol of 3-NT/mol of SERCA2a, i.e. the nitration of more than 20% of all tyrosine residues.
To date, studies investigating the effect of aging on cysteine residues in SERCA in vivo have only provided information about the average number of cysteine residues modified per molecule of protein. However, a detailed mapping of the specific cysteine residues targeted by age-dependent oxidation is of tremendous interest in view of recent studies from our laboratories showing the selective stimulation of SERCA by physiological levels of NO [13]. It is known that biological aging leads to a partial loss of NO-dependent vascular relaxation, and a molecular rationale for this phenomenon could be the age-dependent oxidation of Cys669 and/or Cys674 of smooth muscle SERCA2 [13]. Therefore we have designed MS experiments aimed at achieving a detailed map of SERCA cysteine residues in tissues of young and old animals. For comparison, we also exposed SERCA from young animals to oxidation by peroxynitrite in vitro. Peroxynitrite was selected as an in vitro oxidant on the bases of our earlier results showing that NO- and probably peroxynitrite-dependent modifications play an important role in the oxidative modification of SERCA in vivo [15]. In order to optimize the method with adequate amounts of protein, we have focused our attention on just one isoform (SERCA1).
EXPERIMENTAL
Animals and tissue samples
The research protocol outlined in the present paper has been approved by the University of Kansas Animal Care Facility. Young (5–6-month-old) and old (34-month-old) Fisher 344×Brown Norway F1 hybrid rats were purchased from the National Institute of Aging, from colonies maintained at Harlan Sprague Dawley. The rats were allowed to adapt for 2 weeks after arrival in a 12 h light/12 h dark cycle and were provided with water and food ad libitum. The animals were killed by decapitation, and hindlimb skeletal muscle (fast-twitch fibres) was rapidly removed, frozen immediately in liquid nitrogen and stored at −80 °C.
Isolation of SR
SR vesicles (light fraction) were prepared as described previously [15,17]. SR was divided into 0.1 ml aliquots, frozen rapidly in liquid nitrogen, and stored at −70 °C. Protein concentration was determined using the BCA (bicinchoninic acid) assay using BSA as a standard according to the manufacturer's instructions (Pierce). At no stage of SR preparation did we use any reducing agents in order to maintain the oxidation state of the protein isolated from tissue.
Reaction with peroxynitrite
Peroxynitrite was prepared by the reaction of ozone with cooled aqueous sodium azide as described previously [18]. SR (10 mg/ml of protein) was re-suspended in a buffer containing 20 mM Na2HPO4, 20 mM NaHCO3 and 1 mM diethylenetriaminepenta-acetic acid (pH 7.4). A small volume of stock peroxynitrite was added rapidly while vortex-mixing to reach desired final concentrations of peroxynitrite up to 3 mM. For control experiments, peroxynitrite was added to the buffer 5 min before mixing with SR membranes (reverse-order-of-addition experiment).
SERCA activity assays
Total, Ca2+-dependent and basal ATPase activities of SERCA in SR were determined at 25 °C by the colorimetric assay of Pi in the presence or absence of the calcium ionophore, A23187, as outlined in our previous paper [19]. To assess the Ca2+-dependence of SERCA activity, the assay was conducted in the presence of 1 mM EGTA and 0.2–4 mM CaCl2 to achieve the desired free Ca2+ concentrations as calculated using the CHELATOR program [20]. In all of our experiments, the measured Ca2+-dependent ATP hydrolysis was attributed to SERCA because the Ca2+-ATPase activity was inhibited completely by the addition of 20 μM thapsigargin (Sigma).
Fluorescent labelling of protein thiols
Oxidation of cysteine residues was monitored using the maleimide-based fluorescent dye TG1 {ThioGlo® 1 or methyl-10-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)-9-methoxy-3-oxo-3H-benzo-[f]chromene-2-carboxylate}, purchased from Covalent Associates. A stock solution of TG1 (2 mM) was prepared in acetonitrile. SR samples, containing 100 μg of protein in 20 mM sodium phosphate, were incubated with 200 μM TG1 in the presence of 10% (v/v) acetonitrile and 2% (w/v) SDS for 30 min at 37 °C and pH 7.4 to achieve maximum labelling of SR cysteine residues, as monitored by fluorescent spectroscopy. Fluorescence emission spectra of cysteine–TG1 protein adducts (10 μg of total SR protein) were recorded in 1-ml samples using excitation and emission wavelengths of 379 and 513 nm respectively, using a Shimadzu RF5000U fluorescence spectrophotometer. Glutathione was used to calibrate the absolute amount of thiols in SERCA.
Pyridoethylation of cysteine residues
For detection of reversibly oxidized cysteine residues, SERCA gel bands obtained after SDS/4–20% PAGE separation of TG1-labelled SR were excised and extensively washed with a buffer containing 100 mM NH4HCO3 and 50% (v/v) acetonitrile, incubated with 2 mM DTT (dithiothreitol) for 30 min at 37 °C, followed by incubation with 2% (v/v) 4-VP (4-vinylpyridine) for 1 h at room temperature (20 °C) in the dark. 4-VP was obtained from Sigma and was freshly distilled as described previously [21].
HPLC separation of proteins
The RP-HPLC (reversed-phase HPLC) fractionation of proteins from SR was achieved according to our previously published procedure [22]. Chromatograms were monitored by absorbance (at 280 nm) or by fluorescence detection (at excitation and emission wavelengths of 379 and 513 nm respectively).
Gel electrophoresis
Native and TG1-labelled SR samples were mixed with Tris/glycine/SDS sample buffer and loaded into ten-well 1.5-mm-thick Novex Tris/glycine gradient 4–20% polyacrylamide gels (Invitrogen). After running gel electrophoresis at 200 V for 90 min, the gels were stained by 0.1% Coomassie Blue R250 in ethanoic (acetic) acid/methanol/water (1:3:6, by vol.) for 1 h, and destained in ethanoic acid/methanol/water (1.5:8:10.5, by vol.) until the gel backgrounds were clear.
In-gel proteolytic digestion
Protein bands of interest were excised from the gel and digested with trypsin as described previously [22] before MS analysis.
HPLC–ESI (electrospray ionization)–MS and MS/MS (tandem MS) analysis
Tryptic peptides were separated on a RP-HPLC column (0.32 mm×150 mm Symmetry C18) at a flow rate of 8 μl/min with a linear gradient rising from 20 to 95% (v/v) methanol in 0.08% (v/v) aqueous methanoic (formic) acid over a period of 75 min using a Waters CapLC XE system. Peptides were eluted directly into the source of a Q-TOF2™ mass spectrometer (Micromass) with automatic functional switching between survey MS and MS/MS modes. Peaks with an intensity of more than six counts in a single scan were selected automatically for MS/MS analysis. Identified peptides were then quantified using the MassLinx 4.0 software (Micromass) by the integration of peaks in selected ion chromatograms obtained in additional HPLC–ESI–MS runs.
NMR measurements
1H-NMR spectra were recorded on a Bruker Avance 500 MHz NMR spectrometer. All proton signals were measured relative to TMS (tetramethylsilane) as an external standard in a capillary tube. Stock solutions (2 mM) of TG1 in DMSO-d6 and NAC (N-acetyl-L-cysteine) in a buffer containing 40 mM NaH2PO4 (pH 7.2) in 4:1 (v/v) H2O/2H2O were mixed at a 1:1 ratio and incubated for 30 min at 37 °C to obtain the TG1–NAC adduct. Aliquots (0.6 ml) were incubated at different pH for different times to mimic conditions encountered by TG1-labelled peptides during sample preparation for MS analysis. All NMR spectra were recorded at 25 °C.
Statistical analysis
Quantitative results were obtained from the data for at least three independent experiments involving material isolated from different animals. SR used in the analysis of age-dependent differences was isolated separately from the skeletal muscles of five young (5–6-month-old) and four old (34-month-old) rats. Values are presented as means±S.D. Significance of a difference between two means was assessed using Student's t test, calculated using a two-sample unequal variance and two-tailed distribution using Microsoft Excel XP Pro software.
RESULTS
Validation of the use of TG1 as a quantitative cysteine label for MS analysis
Model experiments were designed to identify products of cysteine labelling with TG1 [molecular mass 379.1 a.m.u. (atomic mass units)] using a double mutant of chicken CaM (calmodulin; Swiss-Prot accession number P62149) that contains two cysteine residues at positions 34 and 110 (CaM T34C/T110C [23]). ESI–MS of the intact reduced CaM T34C/T110C before and immediately after the reaction with TG1 detected a mass increase of 758.5 a.m.u., indicating the stoichiometric incorporation of two equivalents of TG1 (results not shown). Following derivatization, the protein was subjected to trypsin digestion either in solution or in the gel.
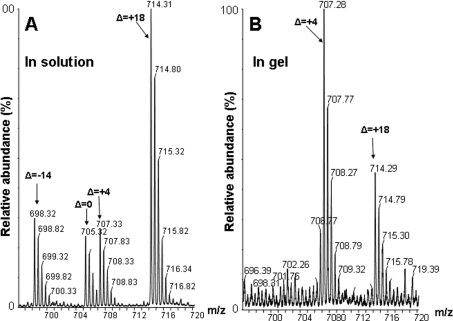
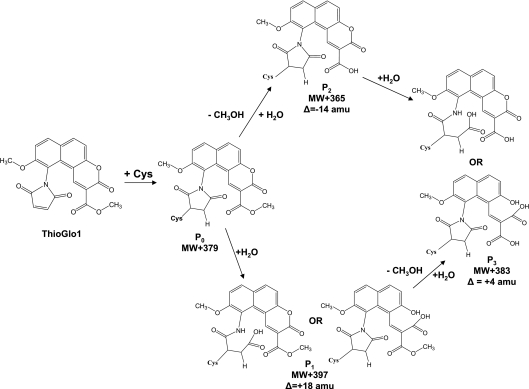
HPLC–ESI–MS/MS analysis of the in-solution-digested sample identified doubly charged ions, [M+2H]2+, with m/z 593.76 and m/z 705.32, which correspond to the TG1 adducts of the peptides ELG34CVMR and HVM110CNLGEK respectively (an increase of +379.1 a.m.u. on the respective cysteine residues). However, even stronger signals were detected for adducts of 18 a.m.u. greater in mass, i.e. +397.1 a.m.u., relative to the unmodified peptide (for example, [M+2H]2+ with m/z 714.31 in Figure 1(A) for the peptide HVM110CNLGEK). This is in accord with earlier publications [24,25], which reported succinimide ring hydrolysis at neutral and slightly alkaline pH. Such a hydrolytic opening of the cysteine–TG1 succinimide ring leads to product P1, displayed in Scheme 1. In addition to the mechanism proposed previously [24,25], our NMR measurements suggest some hydrolysis of the lactone ring at pH 2. This is based on 1H-NMR experiments with a model TG1 adduct of NAC, demonstrating a time-dependent downfield shift of the aromatic protons of TG1 (results not shown). The mass spectrometric analysis of both hydrolysis products shows a single peak for P1 with Δ=+18 a.m.u. relative to the unmodified adduct, P0. Solution digestion also yielded small quantities of additional products of Δ=−14 and Δ=+4 a.m.u. relative to the expected cysteine–TG1 adduct of HVM110CNLGEK (m/z 698.32 and 707.33 respectively) (Figure 1A). Again, model 1H-NMR experiments with the TG1–NAC adduct at alkaline pH (pH 9) were performed to identify the origin of these products. The incubation of TG1–NAC at slightly alkaline pH resulted in the formation of methanol, accompanied by the loss of signal from the methyl ester protons, suggesting that these products can be represented by P2 and P3 respectively (Scheme 1), arising from either methyl ester hydrolysis only or methyl ester hydrolysis combined with succinimide/lactone hydrolysis.
Figure 1. Mass spectra of CaM T34C/T110C mutant digested by trypsin in-solution or in-gel.
ESI–MS spectra of a tryptically digested T34C/T110C mutant of CaM modified with TG1 represent peptide HVM110CNLGEK from in-solution (A) and from in-gel (B) digests: [M+2H]2+ 705.3, 714.3, 698.3 and 707.3 are for cysteine–TG1 P0, P1, P2 and P3 products respectively (see Scheme 1).
Scheme 1. Cysteine–TG1 adduct modification in experimental conditions involving SDS/PAGE, protein visualization and in-gel digestion.
MW, molecular mass.
In-gel digestion also generated significant yields of the ring-opened product P1 with Δ=+18 a.m.u. relative to the theoretical mass of P0, i.e. [M+2H]2+ with m/z 705.32 (Figure 1B). However, under these conditions, ester hydrolysis appears to be a major pathway, indicated by the dominance of the [M+2H]2+ ion with m/z 707.28 (Figure 1B). Hence, for the MS identification of TG1-labelled SERCA peptides in the in-gel digests, we searched for all possible products with peptide masses modified by +379.1, +383.1 and +397.1 a.m.u., and used the major isoforms for quantitative analysis. Comparative studies therefore required processing of protein samples under identical conditions.
Incorporation of the fluorescent TG1 label into SERCA
The labelling conditions were optimized to obtain maximum yields of the fluorescent cysteine–TG1 adduct as described in the Experimental section. In SR enriched with SERCA (approx. 40% relative to total rat skeletal muscle SR protein according to gel densitometry [14,15]), more than 90% of the free protein thiols are located on SERCA [14,26]. Our RP-HPLC analysis of rat SR with absorbance and fluorescence detection showed similar values: approx. 90% of the fluorescence signal was located in the chromatographic SERCA peak (results not shown). Therefore, using TG1 labelling of SR, we can determine the molar content of cysteine residues in the SERCA molecule. Maximal TG1 labelling of SERCA required the presence of 2% (w/v) SDS, which resulted in the derivatization of 85±8 mol of thiol/mg of SR protein or 21±2 mol of reactive cysteine/mol of SERCA1. In contrast, only three to four cysteine residues/mol of SERCA1 were labelled in the absence of SDS, in agreement with a previous study [27]. Rat SERCA1 contains 24 cysteine residues. Based on the presence of at least one functionally important disulphide bridge in SERCA1 [28], we may conclude that we achieved essentially complete labelling of the remaining cysteine residues with TG1.
Loss of reactive cysteine residues in SERCA1 upon treatment of SR with peroxynitrite and during biological aging
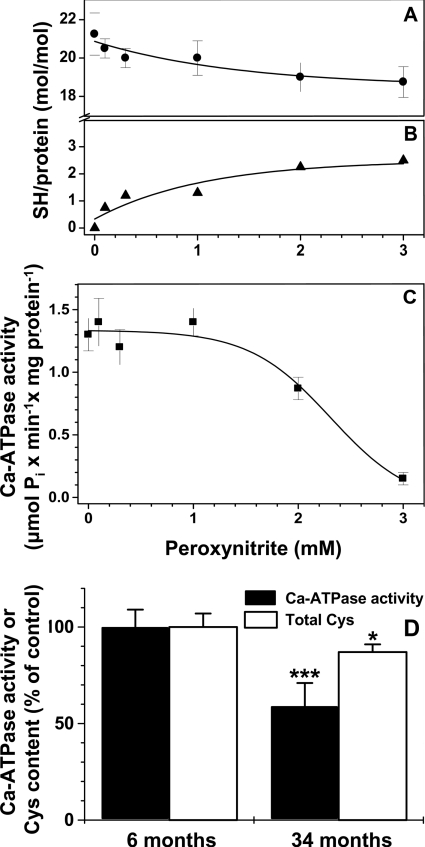
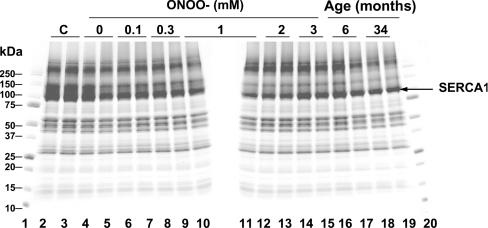
Exposure of SR to peroxynitrite caused a concentration-dependent decrease in the total number of TG1-reactive cysteine residues (Figure 2A). For example, after treatment of SR with 3 mM peroxynitrite, the average number of cysteine residues/mol of SERCA1 susceptible to labelling with TG1 decreased 2.5-fold (Figure 2B). The decrease in the number of reactive cysteine residues was accompanied by a ∼90% inactivation of SERCA (Figure 2C). A comparison of SR from the skeletal muscle of 6- and 34-month-old rats showed a similar decrease in the number of reactive cysteine residues in SERCA1 due to biological aging in vivo, 2.8 mol of cysteine/mol of protein (Figure 2D). However, in contrast with in vitro peroxynitrite inactivation, the age-dependent loss of cysteine residues was accompanied by only a ∼40% SERCA inactivation (1.13±0.13 compared with 1.87±0.17 μmol of Pi/min per mg of protein, for SERCA1 from 34- compared with 6-month-old rats respectively). Treatment with peroxynitrite or labelling with TG1 did not significantly affect the electrophoretic mobility of SERCA1 (Figure 3), permitting us to quantitatively map reactive cysteine residues using SDS/4–20% PAGE separation and in-gel digestion coupled to HPLC–ESI–MS/MS analysis.
Figure 2. Loss of the Ca2+-ATPase activity and reactive cysteine residues in rat skeletal muscle SR after the exposure to peroxynitrite and during biological aging.
(A–C) Total molar content, calculated loss of reactive cysteine residues and Ca2+-ATPase activity (μmol of Pi/min per mg of protein) respectively in 6-month-old rat skeletal muscle SR (5 mg/ml protein in 20 mM sodium phosphate, pH 7.4) after bolus addition of peroxynitrite to the indicated concentrations. (D) Ca2+-ATPase activity (closed bars) and reactive cysteine content (open bars) in SR isolated from young adult (6-month-old) and aged (34-month-old) rat skeletal muscle. Values for 6-month-old rats were set at 100%. Molar content of reactive cysteine was evaluated by the formation of fluorescent cysteine–TG1 adducts in the presence of 2% SDS.
Figure 3. SDS/PAGE of rat skeletal muscle SR after treatment by peroxynitrite in vitro or in vivo aging and TG1 labelling.
Derivatization by TG1 has been performed under non-reducing conditions and in the presence of 2% SDS (except lanes 2 and 3). Lanes 1 and 20, molecular-mass standards (kDa); lanes 2 and 3, intact SR (from 6-month-old rat, non-treated and non-labelled); lanes 4 and 5, reverse-order of 3 mM peroxynitrite addition control; lanes 6 and 7, 0.1 mM; lanes 8 and 9, 0.3 mM; lanes 10 and 11, 1 mM; lanes 12 and 13, 2 mM; lanes 14 and 15, 3 mM peroxynitrite respectively; lanes 16 and 17, SR from 6-month-old rat; lanes 18 and 19, SR from 34-month-old rat.
Mapping and quantification of specific cysteine residues in SERCA
A list of all 19 cysteine-containing tryptic peptides of rat SERCA1 (Swiss-Prot accession number Q64578), ranging in length from 4–88 residues, is displayed in Table 1. Except for four peptides (Table 1, #4, 12, 17 and 19) all peptides were detected by ESI–MS/MS analysis. The largest of these peptides (Table 1, #19) contains three cysteine residues, two of which (Cys876 and Cys888) form a functionally important disulphide bridge in the native protein [28]. The peptides containing cysteine residues at positions 268, 774 and 938 (Table 1, #14, 18 and 16 respectively) were identified as products of TG1 labelling without subsequent hydrolysis of either the ring or the methyl ester (+379.1 a.m.u. relative to native peptide mass). ESI–MS/MS spectra acquired for doubly and triply charged ions, [M+2H]2+ and [M+3H]3+ respectively, identified nine additional peptides containing a single cysteine residue modified by TG1, with both the succinimide or lactone ring and the methyl ester hydrolysed (Δ=+383.1 a.m.u.; see example in Figure 4). These cysteine residues were in positions 12, 364, 377, 471, 498, 525, 561, 614 and 636. The same modification was observed for Cys417 and Cys420, which are located in a single peptide (Table 1, #15). Differential modifications were observed for the two adjacent cysteine residues at positions 674 and 675 (Table 1, #6) with Δ=+383.1 and Δ=+397.1 a.m.u. respectively. Thus a total of 16 cysteine residues that can be labelled with TG1 were observed for SERCA1 under non-reducing conditions. No TG1 labelling was detected for Cys344 or Cys349 (Table 1, #10); this was rationalized by the observation of an intramolecular disulphide bridge under non-reducing conditions (Figure 5), as shown above for Cys876 and Cys888. Four other cysteine-containing peptides involving residues 70, 318, 670 and 910 (Table 1, #1, 12, 17 and 19 respectively) were not observed in any form.
Table 1. HPLC–ESI–MS/MS identification of TG1-labelled cysteine residues in rat SERCA1.
Theoretical mass is for cysteine–TG1 adduct with a mass gain of 379.1 a.m.u. Rt, elution time of a peptide; n.f., not found.
| Native rat SERCA1 cysteine-containing tryptic peptides | TG1 adducts of the peptides | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| # | Start | End | Cysteine | Sequence | Mass* (a.m.u.) | Theoretical mass | Detected mass | Actual m/z | Rt (min) |
| 1 | 668 | 671 | 670 | EACR | 477.2 | 856.3 | n.f. | ||
| 2 | 525 | 529 | 525 | CNYVR | 653.3 | 1032.4 | 1036.4 | 519.2 | 23 |
| 3 | 561 | 567 | 561 | CLALATR | 746.4 | 1125.5 | 1129.5 | 565.8 | 28 |
| 4 | 630 | 637 | 636 | GTAIAICR | 803.4 | 1182.5 | 1186.5 | 594.3 | 27 |
| 5 | 468 | 476 | 471 | ANACNSVIR | 946.5 | 1325.5 | 1329.4 | 665.8 | 25 |
| 6 | 673 | 678 | 674, 675 | ACCFAR | 669.3 | 1427.4 | 1449.4 | 725.7 | 28 |
| 7 | 493 | 502 | 498 | SMSVYCSPAK | 1071.5 | 1450.6 | 1454.5 | 728.3 | 26 |
| 8 | 606 | 615 | 614 | EVTGSIQLCR | 1104.6 | 1483.6 | 1487.6 | 744.8 | 27 |
| 9 | 353 | 365 | 364 | TGTLTTNQMSVCK | 1382.7 | 1761.7 | 1765.7 | 883.9 | 27 |
| 10 | 335 | 352 | 344, 349 | SLPSVETLGCTSVICSDK | 1837.9 | 2595.0† | 1835.8 | 918.9 | 28 |
| 11 | 8 | 30 | 12 | STEECLSYFGVSETTGLTPDQVK | 2490.2 | 2869.2 | 2873.2 | 958.8 | 39 |
| 12 | 298 | 324 | 318 | IAVALAVAAIPEGLPAVITTCLALGTR | 2603.5 | 2982.6 | n.f. | ||
| 13 | 372 | 397 | 377 | VDGDICSLNEFSITGSTYAPEGEVLK | 2743.3 | 3122.4 | 3126.4 | 1043.2 | 42 |
| 14 | 263 | 290 | 268 | VISLICVAVWLINIGHFNDPVHGGSWFR | 3148.7 | 3527.7 | 3527.6 | 882.9 | 55 |
| 15 | 404 | 431 | 417, 420 | AGQYDGLVELATICALCNDSSLDFNETK | 2998.4 | 3756.5 | 3769.8 | 1257.6 | 61 |
| 16 | 925 | 958 | 938 | MPPWVNIWLLGSICLSMSLHFLILYVDPLPMIFK | 3985.1 | 4364.2 | 4365.0 | 1092.3 | 71 |
| 17 | 64 | 110 | 70 | ILLLAACISFVLAWFEEGEETVTAFVEPFVILLILIANAIV | 5244.9 | 5623.9 | n.f. | ||
| GVWQER | |||||||||
| 18 | 763 | 822 | 774 | YLISSNVGEVVCIFLTAALGLPEALIVQLLWVNLVTDGL | 6430.6 | 6809.0 | 6809.5 | 1362.9 | 86 |
| PATALGFNPPDLDIMDRPPR | |||||||||
| 19 | 837 | 924 | 876, 888, 910 | YMAIGGYVGAATVGAAAWWFLYAEDGPHVSYHQLTH | 9742.1 | 10119.2‡ | n.f. | ||
| FMQCTEHNPEFDGLDCEVFEAPEPMTMALSVLVTIEMC | |||||||||
| NALNSLSENQSLLR | |||||||||
* Monoisotopic and average masses for peptides below and above 3500 a.m.u. respectively.
† An internal disulphide mass detected in non-reducing conditions is displayed.
‡ Calculated mass corresponds to addition of only one equivalent of TG1, assuming that Cys876 and Cys888 form a disulphide bond [28].
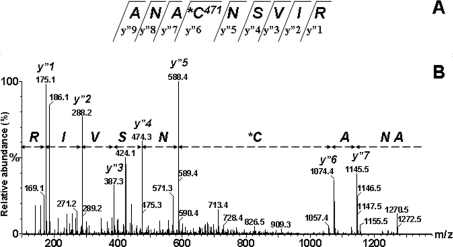
Figure 4. ESI–MS/MS spectrum of the [M+2H]2+ 665.7 ion corresponding to the ANA471CNSVIR, rat SERCA1 tryptic peptide derivatized by TG1.
(A) Fragment y” type ions are indicated in the peptide sequence. (B) The interval from the y”6 (1074.4) peak to the y”5 (588.4) peak is 486.0. This difference is consistent with the cysteine derivatized as TG1 plus water minus the methyl ester (Δ=+4 a.m.u.).
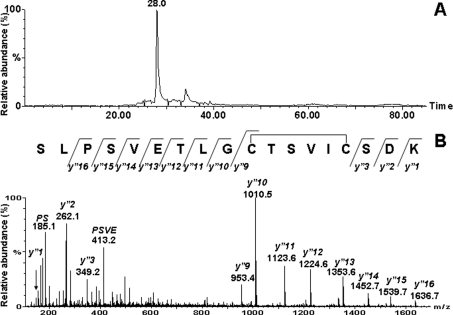
Figure 5. Selected mass chromatogram (A) and ESI–MS/MS spectrum (B) of [M+2H]2+ 918.9.
Inset in (B) shows the sequence of rat SERCA1 tryptic peptide 335–352 (SLPSVETLGC344TSVIC349SDK), in which Cys344 and Cys349 are disulphide-bonded, with detected y”-type fragment ions.
We verified that TG1 labelling of SERCA1 was nearly complete by application of a double-labelling approach. After primary labelling with TG1, protein samples were reduced with DTT and alkylated by 4-VP. HPLC–ESI–MS analysis of the respective tryptic digest did not reveal any significant amounts of cysteine–4-VP (+105 a.m.u.) adducts for cysteine-containing peptides that had been labelled with TG1. In addition, 4-VP labelling was not detected for peptides containing cysteine residues at positions 70, 318, 670, 910, which had not been identified in tryptic digests following TG1 modification. In contrast, the peptide containing the Cys344–Cys349 disulphide bond was labelled successfully with two equivalents of 4-VP. This double-labelling strategy, together with the nearly complete TG1 labelling of SERCA1 indicated by the fluorescence yields, confirmed the high sensitivity of SERCA1 cysteine residues towards TG1 in the presence of 2% SDS. The failure to detect some cysteine residues using our ESI–MS/MS approach can therefore be attributed to poor recovery of the peptides from either the gel or the HPLC column. Indeed, three of the missing cysteine residues (70, 318 and 910) are located on transmembrane helices that are highly hydrophobic and devoid of trypsin cleavage sites. In order to test a potential effect of oxidized cysteine residues, e.g. intramolecular disulphide bridges, on protein conformation, which may affect the efficiency of SERCA1 labelling and peptide yields, we compared the effect of DTT reduction on SR samples from both young and old animals. No significant changes in the yields of TG1-labelled peptides between the respective reduced and native samples were detected, except for the peptide originally containing the Cys344–Cys349 disulphide bond.
For the direct comparison of SERCA from different tissue samples, e.g. young and old muscle, care was taken that samples were run on the same gel in order to minimize variations arising from sample processing, i.e. times for electrophoresis, staining/destaining, etc. Gel bands were also processed in parallel tubes under identical conditions. Moreover, a set of internal standards was employed for the quantitative comparison of protein samples, as described below.
Internal standards for TG1 labelling and peptide recovery
For quantitative analysis, a set of internal standards was selected, consisting of seven rat SERCA1 tryptic peptides, which lack oxidation- and hydrolysis-sensitive amino acids such as cysteine, methionine, tryptophan, tyrosine, histidine and asparagine (Table 2). All TG1-containing peptides were normalized to the yields of these seven standard peptides in order to account for potential variations owing to differences in the digestion yields between protein samples and peptide recovery from the gel or HPLC column, which may be a function of sequence modifications and/or conformational changes of the protein. Normalization coefficients were obtained by averaging the yields of these seven standard peptides. Statistical analysis showed that the S.D. of these normalization coefficients was usually within 15–20%.
Table 2. SERCA1 tryptic peptides used as internal standards for quantitative mapping of reactive cysteine residues.
Peptide sequences were confirmed by MS/MS analysis.
| Sequence | Start position | End position | Peptide mass (a.m.u.) | Actual m/z | Charge state |
|---|---|---|---|---|---|
| VPLTGPVK | 535 | 542 | 809.5 | 810.5 | +1 |
| EFTLEFSR | 482 | 489 | 1027.5 | 1028.5 | +1 |
| EFDDLPLAEQR | 657 | 667 | 1331.6 | 666.8 | +2 |
| AVGIVATTGVSTEIGK | 219 | 234 | 1501.8 | 751.9 | +2 |
| DIVPGDIVEVAVGDK | 144 | 158 | 1524.8 | 763.4 | +2 |
| VGEATETALTTLVEK | 437 | 451 | 1560.8 | 781.4 | +2 |
| VDQSILTGESVSVIK | 175 | 189 | 1573.9 | 787.9 | +2 |
Loss of specific cysteine residues upon biological aging in vivo or exposure to peroxynitrite in vitro
Table 3 displays the quantitative results on the relative loss of specific cysteine residues in SERCA1. Treatment with up to 3 mM peroxynitrite resulted in a statistically significant (90% confidence limits) partial loss of six out of 16 displayed reactive cysteine residues. In contrast, biological aging resulted in a partial loss of nine cysteine residues. Five cysteine residues (525, 674, 675, 498 and 938) were modified both in vivo and in vitro; Cys364, Cys417 and Cys420 were affected only by peroxynitrite in vitro while Cys561, Cys636, Cys614, Cys377 and Cys774 were partially lost during in vivo aging. Importantly, the total losses of cysteine for peroxynitrite-treated and aging SR, calculated from the integration of the mass spectrometric data, 2.57 and 3.18 mol of cysteine/mol of SERCA, were close to the values obtained by fluorescence spectroscopy of intact TG1-labelled SERCA, 2.5 and 2.8 mol of cysteine/mol of SERCA respectively. This excellent agreement validated the quantitative mass spectrometric approach. This agreement also means that the remaining cysteine residues, which were not resolved by quantitative MS, most probably do not contribute to the measured total loss of reactive cysteine in SERCA.
Table 3. Content of individual cysteine residues in rat skeletal muscle SERCA1 (mol of cysteine/mol of protein) after exposure to peroxynitrite in vitro or resulting from biological aging in vivo.
| TG1-labelled cysteine content in SERCA1 (ratio to control) | ||||||
|---|---|---|---|---|---|---|
| Peroxynitrite (mM) | Statistically significant loss of TG1-labelled cysteine residues† | |||||
| Cysteine sequence position | 0.3 | 1 | 3 | Aging (34 months) | Peroxynitrite (3 mM) | Aging |
| 525 | 0.66±0.13* | 0.74±0.07* | 0.69±0.07* | 0.73±0.12* | 0.31 | 0.27 |
| 561 | 0.87±0.05 | 0.91±0.06 | 0.81±0.03 | 0.55±0.16** | − | 0.45 |
| 636 | 0.86±0.04 | 0.87±0.03 | 0.75±0.08 | 0.65±0.10** | − | 0.35 |
| 471 | 0.94±0.08 | 0.89±0.12 | 0.90±0.05 | 1.15±0.30 | − | − |
| 674, 675 | 0.80±0.13 | 0.79±0.12 | 0.63±0.05* | 0.60±0.20** | 0.37 | 0.40 |
| 498 | 0.82±0.01 | 0.77±0.08* | 0.56±0.04* | 0.69±0.27* | 0.44 | 0.31 |
| 614 | 0.90±0.06 | 0.94±0.14 | 0.87±0.04 | 0.54±0.07** | − | 0.46 |
| 364 | 0.78±0.01 | 0.65±0.03* | 0.41±0.07** | 0.70±0.36 | 0.59 | − |
| 12 | 0.98±0.14 | 0.91±0.13 | 1.00±0.18 | 1.29±0.53 | − | − |
| 377 | 1.02±0.21 | 1.01±0.19 | 1.06±0.20 | 0.61±0.11** | − | 0.27 |
| 268 | 0.75±0.41 | 1.10±0.46 | 1.13±0.28 | 1.07±0.29 | − | − |
| 417, 420 | 0.62±0.09 | 0.55±0.05** | 0.49±0.01** | 1.10±0.26 | 0.51 | − |
| 938 | 0.83±0.22 | 0.63±0.33 | 0.35±0.10** | 0.63±0.21** | 0.65 | 0.37 |
| 774 | 0.99±0.52 | 1.34±0.72 | 1.35±0.42 | 0.70±0.22* | − | 0.30 |
| Total loss‡ | 0.34 | 1.29 | 2.57 | 3.18 | 2.57 | 3.18 |
† Loss is calculated and shown only for a statistically significant (*, 90% confidence limits; **, 95% confidence limits) decrease in an individual cysteine residue content, using values measured for SR samples from 6-month-old animals as a control (1.0±0.15).
‡ Total loss is calculated as the sum of the statistically significant individual losses for all displayed cysteine residues.
Effects of biological aging and peroxynitrite on Ca2+ and ATP affinity
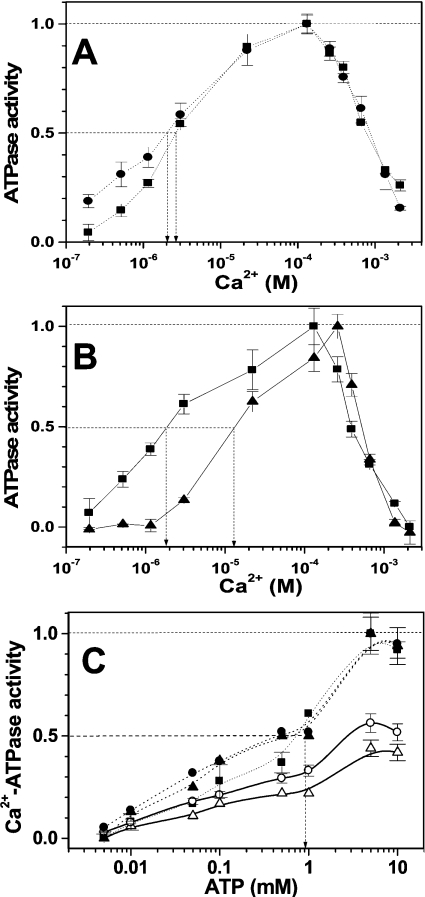
For skeletal muscle SR from 5–6-month-old rats (0.6 mg/ml protein), an increase in the concentration of added Ca2+ from 0.2 to 1.2 mM (corresponding to 0.19 μM and 0.132 mM free Ca2+ respectively in the presence of 1 mM EGTA and other buffer components) stimulated the SERCA activity more than 20-fold, showing that the protein in the SR preparation from young rats is functionally and structurally intact (Figures 6A and 6B). Biological aging in vivo and treatment with peroxynitrite in vitro affected the Ca2+ concentration-dependence in different ways. SR from 34-month-old animals exhibited ∼40% lower ATPase activity compared with SR from 5–6-month-old animals at free Ca2+ concentrations of 22 μM used for Ca2+-ATPase activity measurements. Importantly, the analysis of normalized curves (Figure 6A) does not show any significant difference in the Ca2+ concentration for half-maximal activation (K0.5 ∼2 μM under our conditions), which characterizes the apparent Ca2+ affinity of SERCA in the E1 conformational state [1,29]. Therefore the affinity of SERCA to Ca2+ does not significantly change with age. However, at low Ca2+ concentrations (below 1.16 μM), the shape of the dependence for SR from old animals is different, reflecting a lower structural and functional integrity of ‘old’ SERCA. An increase in the concentration of free Ca2+ stimulated the ATPase SERCA activity only 5-fold owing to a higher relative contribution of Ca2+-independent (basal) ATPase activity in the SR from old compared with young animals (Figure 6A). Peroxynitrite produced a significant decrease in the Ca2+ affinity (Figure 6B): K0.5 changed from 2 to 10 μM, reflecting the alteration of either Ca2+-binding domains or domains involved in allosteric regulation of the high-affinity Ca2+-binding sites, as was demonstrated for phospholamban and sarcolipin [29,30]. Analysis of the ATP dependence of Ca2+-ATPase activity (Figure 6C) did not show age-associated differences, and no effect of peroxynitrite on the ATP affinity of rat SERCA1 (K0.5∼1 mM in our experiments).
Figure 6. Effect of peroxynitrite and biological aging on Ca2+ and ATP affinity of rat skeletal muscle SERCA1.
(A and B) The dependences of ATPase activity on free (calculated) Ca2+ concentration. (C) ATP concentration dependences of Ca2+-ATPase activity. SR samples were from 5–6-month-old rat without (squares) or after treatment by 300 μM peroxynitrite (triangles), and from 34-month-old animals (circles). Closed symbols, values normalized to maximum for each concentration curve; open symbols, values relative to control (i.e. intact 5–6-month-old SR at free Ca2+ concentration of 22.2 μM used for Ca2+-ATPase activity determination). Arrows indicate the determination of respective half-maximal activation concentrations, K0.5.
DISCUSSION
The redox state of specific cysteine residues of SERCA is important for enzymatic function so that modifications of different SERCA cysteine residues may result in both inhibition and activation of the protein [13,14,31]. Earlier studies on the role of cysteine modifications in the regulation of SERCA activity were based on either selective labelling or site-directed mutagenesis of cysteine residues that resulted in a significant change in protein activity [13,31–36]. Although these approaches specifically addressed SERCA modifications of selected cysteine residues, they are not so relevant to oxidative modifications of the protein cysteine residues in vivo. Previous studies involving proteolytic mapping of SERCA and HPLC–MS analysis detected several specific cysteine-containing peptides with a mass increase fitting to sulphoxidation, nitrosation and S-glutathiolation of SERCA cysteine residues, and quantified the loss of some cysteine residues upon oxidation in vitro using reactive thiol labelling [13,14,33]. However, all previous studies suffered from both incomplete cysteine labelling in SERCA and the use of peptide masses only (MS1 mode), but not MS/MS, for the identification of labelled peptides. Moreover, these earlier studies did not use modification-resistant peptides as internal standards for digestion yields of cysteine-containing peptides. The approach described in the present paper overcomes these shortcomings. First, labelling of the SR with TG1 in 2% SDS resulted in virtually complete derivatization of reduced cysteine residues of SERCA1. Secondly, in-gel processing of the protein allowed the recovery of most of the cysteine-containing peptides, and of additional peptides, which can be used to normalize the yields of the labelled peptides by quantitative HPLC–ESI–MS/MS. The quantitative analysis of the TG1-labelled sequences provides a differential display of 16 out of 20 reduced cysteine residues in SERCA1 available for oxidative modification. A quantitative mapping of the specific cysteine residues in rat skeletal muscle tissue revealed nine cysteine residues targeted by age-dependent oxidation in vivo and six cysteine residues partially lost upon peroxynitrite treatment of the SR in vitro. Importantly, quantification of total cysteine losses by HPLC–MS analysis of SERCA1 digests is in agreement with the data obtained by fluorescence spectroscopy of the intact TG1-labelled protein, validating our approach and permitting the analysis of a role for specific cysteine residues in the control of SERCA activity. A comparative analysis of individual cysteine oxidation and Ca2+-ATPase activity shows that not all the oxidation-sensitive cysteine residues are equally important for SERCA function. A comparison of data from Table 3 with Figure 2(C) illustrates that the peroxynitrite-dependent loss of individual cysteine residues coincides with the loss of SERCA activity only for the residues at positions 674/675 and 938. No other cysteine residue shows the characteristic lag phase correlating with protein inactivation. It is likely that some of the earlier targeted cysteine residues, i.e. at positions 525, 498, 417, 420 and 364, actually protect functionally important cysteine residues from oxidation and could be considered to be intramolecular antioxidants similar to the function ascribed to protein methionine residues [14,37]. At present, we cannot state whether the oxidation of Cys674, Cys675 or Cys938 alone serves as a ‘redox switch’ for SERCA1 activity, or the loss of the protein activity is because of a combination of multiple cysteine oxidation events and/or additional modifications such as methionine oxidation and tyrosine nitration. Various mechanisms for the peroxynitrite modification of thiols have been described, which may yield nitrosothiols, nitrothiols, disulphides, and sulphenic and sulphonic acid [14,38,39]; our labelling technique alone cannot specify the nature of the respective cysteine oxidation products of SERCA. Biological aging leads to a partial loss of SERCA1 activity in rat skeletal muscle (Figure 2D), and a molecular rationale for this phenomenon could be the age-dependent oxidation of specific cysteine residues by peroxynitrite and/or other endogenous oxidants. Interestingly, the affected residues in vivo do not completely match those targeted by peroxynitrite in vitro (Table 3). Both peroxynitrite in vitro and aging in vivo target cysteine residues at positions 525, 498, 674/675 and 938. However, peroxynitrite also targets Cys364, Cys417 and Cys420, whereas aging additionally affects Cys377, Cys561, Cys614 and Cys774. The modification of these latter residues appears not to contribute significantly to the age-dependent loss of SERCA function. This observation possibly reflects the occurrence and reaction of additional oxidants in vivo. In addition, the conformation of SERCA in vitro may not be completely identical with that in vivo, a feature which may lead to some differential reaction kinetics of cysteine in vitro compared with in vivo. Moreover, not only the loss of a specific cysteine residue, but also the nature of its specific cysteine oxidation product, will determine its ultimate effect on protein function.
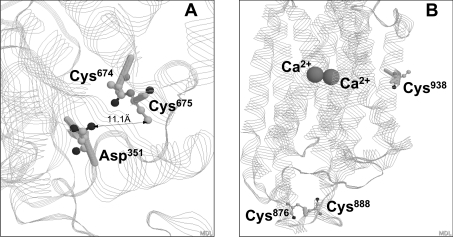
At present, we cannot conclude which cysteine residue is more important for the loss of SERCA1 activity observed in aging rat skeletal muscle. Our ligand-binding studies did not show significant changes in either the Ca2+ or ATP affinity of SERCA1 in aging SR. In contrast, the inactivation of SR by peroxynitrite in vitro is accompanied by a decrease in Ca2+ affinity (Figure 6C). These data, again, demonstrate that SERCA cysteine oxidation by peroxynitrite in vitro does not completely simulate the age-dependent modification. We did not detect a modification of cysteine residues vicinal to the high-affinity Ca2+-binding sites, suggesting that the effect of cysteine oxidation on catalytic function may not be due to modification of specific ligand binding sites on the protein, but, rather, through more general effects on the protein structure, uncoupling ATP hydrolysis from Ca2+ translocation. In support of this suggestion are our data showing that the decrease in Ca2+-dependent ATPase activity in aging SR results merely from an increasing contribution of the Ca2+-independent (‘basal’) component of the total ATPase activity (Figure 6A). Figure 7 illustrates the location of Cys674, Cys675 and Cys938 relative to functionally important sites of SERCA1. In the three-dimensional SERCA1 structure, Cys674 and Cys675 are located in the cytosolic part of the protein proximal to the phosphorylation site Asp351 (Figure 7A). In contrast, Cys938 is located on the protein–lipid membrane interface of the transmembrane α-helix M9 (Figure 7B), which is far from specific ligand-binding domains. However, its potential involvement in protein–lipid interactions within the SR membrane may be important for the overall protein structure and specific activity. Previously, our MS studies on rabbit SERCA1 suggested an involvement of Cys349 in the oxidative inactivation of the protein. In the present study, we cannot account for Cys349, because the TG1-labelled peptide containing both Cys344 and Cys349 was not recovered either from the gel or from the HPLC column. Instead we only recovered the intra-disulphide-linked peptide (see above). However, the nearly quantitative correlation of TG1-labelled peptides recovered for MS analysis with the global fluorescence spectroscopic analysis of the complete TG1-labelled SERCA1 suggests that, under the current experimental conditions, Cys349 represents a minor target for peroxynitrite. This difference between rabbit and rat SERCA may originate from experimental differences, such as protein loading of the SR (72% SERCA in rabbit compared with 40% SERCA in rat), which may control, for example, the well-known non-covalent dimerization [40] of SERCA in the SR. Our earlier studies demonstrated an important role of Cys674 for the NO-dependent activation of SERCA at physiological levels of NO [13]. The age-dependent loss of Cys674 suggests that this NO-dependent pathway may be impaired in the aging phenotype.
Figure 7. Location of cysteine residues potentially involved in the control of Ca2+-ATPase activity in the three-dimensional structure of rabbit SERCA1 (PDB code 1SU4).
(A) Location of Cys674 and Cys675 respective to a phosphorylation site, Asp351 (magnified). (B) Location of Cys938 in the transmembrane α-helix M9. Positions of two Ca2+ ions bound to high-affinity sites and of the intramolecular disulphide bridge, C876–C888, are also shown. The highlighted amino acid residues are shown in the CPK (Corey–Pauling–Koltun) (element) code using Protein Explorer 2.45 software.
Acknowledgments
This work was supported by a grant from the NIH (National Institutes of Health) (P01AG12993). The Q-TOF2 was purchased with support from NSF (National Science Foundation) EPSCoR (Experimental Program to Stimulate Competitive Research) and the University of Kansas. The Waters CapLC was purchased for the University of Kansas by the Kansas City Area Life Sciences Institute. We thank Dr D. Vandervelde and S. Neuenswander for their assistance with the NMR measurements and the interpretation of the results.
References
- 1.MacLennan D. H., Rice W. J., Green N. M. The mechanism of Ca2+ transport by sarco(endo)plasmic reticulum Ca2+-ATPases. J. Biol. Chem. 1997;272:28815–28828. doi: 10.1074/jbc.272.46.28815. [DOI] [PubMed] [Google Scholar]
- 2.East J. M. Sarco(endo)plasmic reticulum calcium pumps: recent advances in our understanding of structure/function and biology. Mol. Membr. Biol. 2000;17:189–200. doi: 10.1080/09687680010009646. [DOI] [PubMed] [Google Scholar]
- 3.Zwaal R. R., Van Baelen K., Groenen J. T., van Geel A., Rottiers V., Kaletta T., Dode L., Raeymaekers L., Wuytack F., Bogaert T. The sarco-endoplasmic reticulum Ca2+ ATPase is required for development and muscle function in Caenorhabditis elegans. J. Biol. Chem. 2001;276:43557–43563. doi: 10.1074/jbc.M104693200. [DOI] [PubMed] [Google Scholar]
- 4.Cho J. H., Bandyopadhyay J., Lee J., Park C. S., Ahnn J. Two isoforms of sarco/endoplasmic reticulum calcium ATPase (SERCA) are essential in C. elegans. Gene. 2000;261:211–219. doi: 10.1016/s0378-1119(00)00536-9. [DOI] [PubMed] [Google Scholar]
- 5.Gelebart P., Kovacs T., Brouland J. P., van Gorp R., Grossmann J., Rivard N., Panis Y., Martin V., Bredoux R., Enouf J., Papp B. Expression of endomembrane calcium pumps in colon and gastric cancer cells: induction of SERCA3 expression during differentiation. J. Biol. Chem. 2002;277:26310–26320. doi: 10.1074/jbc.M201747200. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y. P., Teng D., Dralyuk F., Ostrega D., Roe M. W., Philipson L., Polonsky K. S. Apoptosis in insulin-secreting cells: evidence for the role of intracellular Ca2+ stores and arachidonic acid metabolism. J. Clin. Invest. 1998;101:1623–1632. doi: 10.1172/JCI1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanoverberghe K., Vanden Abeele F., Mariot P., Lepage G., Roudbaraki M., Bonnal J. L., Mauroy B., Shuba Y., Skryma R., Prevarskaya N. Ca2+ homeostasis and apoptotic resistance of neuroendocrine-differentiated prostate cancer cells. Cell Death Differ. 2004;11:321–330. doi: 10.1038/sj.cdd.4401375. [DOI] [PubMed] [Google Scholar]
- 8.MacLennan D. H. Ca2+ signalling and muscle disease. Eur. J. Biochem. 2000;267:5291–5297. doi: 10.1046/j.1432-1327.2000.01566.x. [DOI] [PubMed] [Google Scholar]
- 9.Papp B., Brouland J. P., Gelebart P., Kovacs T., Chomienne C. Endoplasmic reticulum calcium transport ATPase expression during differentiation of colon cancer and leukaemia cells. Biochem. Biophys. Res. Commun. 2004;322:1223–1236. doi: 10.1016/j.bbrc.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Prasad V., Okunade G. W., Miller M. L., Shull G. E. Phenotypes of SERCA and PMCA knockout mice. Biochem. Biophys. Res. Commun. 2004;322:1192–1203. doi: 10.1016/j.bbrc.2004.07.156. [DOI] [PubMed] [Google Scholar]
- 11.Bobe R., Bredoux R., Corvazier E., Andersen J. P., Clausen J. D., Dode L., Kovacs T., Enouf J. Identification, expression, function, and localization of a novel (sixth) isoform of the human sarco/endoplasmic reticulum Ca2+-ATPase 3 gene. J. Biol. Chem. 2004;279:24297–24306. doi: 10.1074/jbc.M314286200. [DOI] [PubMed] [Google Scholar]
- 12.Dode L., Vilsen B., Van Baelen K., Wuytack F., Clausen J. D., Andersen J. P. Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 1 and 3 isoforms by steady-state and transient kinetic analyses. J. Biol. Chem. 2002;277:45579–45591. doi: 10.1074/jbc.M207778200. [DOI] [PubMed] [Google Scholar]
- 13.Adachi T., Weisbrod R. M., Pimentel D. R., Ying J., Sharov V. S., Schöneich C., Cohen R. A. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 14.Viner R. I., Williams T. D., Schöneich C. Peroxynitrite modification of protein thiols: oxidation, nitrosilation, and S-glutathiolation of functionally important cysteine residue(s) in the sarcoplasmic reticulum Ca-ATPase. Biochemistry. 1999;38:12408–12415. doi: 10.1021/bi9909445. [DOI] [PubMed] [Google Scholar]
- 15.Viner R. I., Ferrington D. A., Williams T. D., Bigelow D. J., Schöneich C. Protein modification during biological aging: selective tyrosine nitration of the SERCA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle. Biochem. J. 1999;340:657–669. [PMC free article] [PubMed] [Google Scholar]
- 16.Knyushko T. V., Sharov V. S., Williams T. D., Schöneich C., Bigelow D. J. 3-Nitrotyrosine-modification of SERCA2a in the aging heart: a distinct signature of cellular redox environment. Biochemistry. 2005;44:13071–13081. doi: 10.1021/bi051226n. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez J. L., Rosemblatt M., Hidalgo C. Highly purified sarcoplasmic reticulum vesicles are devoid of Ca2+-independent (‘basal’) ATPase activity. Biochim. Biophys. Acta. 1980;599:552–568. doi: 10.1016/0005-2736(80)90199-6. [DOI] [PubMed] [Google Scholar]
- 18.Pryor W. A., Cueto R., Jin X., Koppenol W. H., Ngu-Schwemlein M., Squadrito G. L., Uppu P. L., Uppu R. M. A practical method for preparing peroxynitrite solutions of low ionic strength and free of hydrogen peroxide. Free Radical Biol. Med. 1995;18:75–83. doi: 10.1016/0891-5849(94)00105-s. [DOI] [PubMed] [Google Scholar]
- 19.Dremina E. S., Sharov V. S., Kumar K., Zaidi A., Michaelis E. K., Schöneich C. Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) Biochem. J. 2004;383:361–370. doi: 10.1042/BJ20040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenmakers T. J., Visser G. J., Flik G., Theuvenet A. P. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992;12:870–879. [PubMed] [Google Scholar]
- 21.Sechi S., Chait B. T. Modification of cysteine residues by alkylation: a tool in peptide mapping and protein identification. Anal. Chem. 1998;70:5150–5158. doi: 10.1021/ac9806005. [DOI] [PubMed] [Google Scholar]
- 22.Sharov V. S., Galeva N. A., Knyushko T. V., Bigelow D. J., Williams T. D., Schöneich C. Two-dimensional separation of the membrane protein sarcoplasmic reticulum Ca-ATPase for high-performance liquid chromatography-tandem mass spectrometry analysis of posttranslational protein modifications. Anal. Biochem. 2002;308:328–335. doi: 10.1016/s0003-2697(02)00261-0. [DOI] [PubMed] [Google Scholar]
- 23.Allen M. W., Urbauer R. J., Zaidi A., Williams T. D., Urbauer J. L., Johnson C. K. Fluorescence labeling, purification, and immobilization of a double cysteine mutant calmodulin fusion protein for single-molecule experiments. Anal. Biochem. 2004;325:273–284. doi: 10.1016/j.ab.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 24.Osborn K. D., Bartlett R. K., Mandal A., Zaidi A., Urbauer R. J., Urbauer J. L., Galeva N., Williams T. D., Johnson C. K. Single-molecule dynamics reveal an altered conformation for the autoinhibitory domain of plasma membrane Ca2+-ATPase bound to oxidatively modified calmodulin. Biochemistry. 2004;43:12937–12944. doi: 10.1021/bi048806p. [DOI] [PubMed] [Google Scholar]
- 25.Majima E., Goto S., Hori H., Shinohara Y., Hong Y. M., Terada H. Stabilities of the fluorescent SH-reagent eosin-5-maleimide and its adducts with sulfhydryl compounds. Biochim. Biophys. Acta. 1995;1243:336–342. doi: 10.1016/0304-4165(94)00159-u. [DOI] [PubMed] [Google Scholar]
- 26.Murphy A. J. Sulfhydryl group modification of sarcoplasmic reticulum membranes. Biochemistry. 1976;15:4492–4496. doi: 10.1021/bi00665a025. [DOI] [PubMed] [Google Scholar]
- 27.Hua S., Fabris D., Inesi G. Characterization of calcium, nucleotide, phosphate, and vanadate bound states by derivatization of sarcoplasmic reticulum ATPase with ThioGlo1. Biophys. J. 1999;77:2217–2225. doi: 10.1016/S0006-3495(99)77062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toyoshima C., Nakasako M., Nomura H., Ogawa H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature (London) 2000;405:647–655. doi: 10.1038/35015017. [DOI] [PubMed] [Google Scholar]
- 29.Toyofuku T., Kurzydlowski K., Lytton J., MacLennan D. H. The nucleotide binding/hinge domain plays a crucial role in determining isoform-specific Ca2+ dependence of organellar Ca2+-ATPases. J. Biol. Chem. 1992;267:14490–14496. [PubMed] [Google Scholar]
- 30.MacLennan D. H., Asahi M., Tupling A. R. The regulation of SERCA-type pumps by phospholamban and sarcolipin. Ann. N.Y. Acad. Sci. 2003;986:472–480. doi: 10.1111/j.1749-6632.2003.tb07231.x. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Camacho P. Ca2+-dependent redox modulation of SERCA 2b by ERp57. J. Cell Biol. 2004;164:35–46. doi: 10.1083/jcb.200307010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawakita M., Yamashita T. Reactive sulfhydryl groups of sarcoplasmic reticulum ATPase. III. Identification of cysteine residues whose modification with N-ethylmaleimide leads to loss of the Ca2+-transporting activity. J. Biochem. (Tokyo) 1987;102:103–109. doi: 10.1093/oxfordjournals.jbchem.a122021. [DOI] [PubMed] [Google Scholar]
- 33.Velasco-Guillen I., Guerrero J. R., Gomez-Fernandez J. C., Teruel J. A. Labeling the Ca2+-ATPase of skeletal muscle sarcoplasmic reticulum with maleimidylsalicylic acid. J. Biol. Chem. 2000;275:39103–39109. doi: 10.1074/jbc.M001871200. [DOI] [PubMed] [Google Scholar]
- 34.Viner R. I., Williams T. D., Schöneich C. Nitric oxide-dependent modification of the sarcoplasmic reticulum Ca-ATPase: localization of cysteine target sites. Free Radical Biol. Med. 2000;29:489–496. doi: 10.1016/s0891-5849(00)00325-7. [DOI] [PubMed] [Google Scholar]
- 35.Bishop J. E., Squier T. C., Bigelow D. J., Inesi G. (Iodoacetamido)fluorescein labels a pair of proximal cysteines on the Ca2+-ATPase of sarcoplasmic reticulum. Biochemistry. 1988;27:5233–5240. doi: 10.1021/bi00414a043. [DOI] [PubMed] [Google Scholar]
- 36.Yamashita T., Kawakita M. Reactive sulfhydryl groups of sarcoplasmic reticulum ATPase. II. Site of labeling with iodoacetamide and its fluorescent derivative. J. Biochem. (Tokyo) 1987;101:377–385. doi: 10.1093/oxfordjournals.jbchem.a121922. [DOI] [PubMed] [Google Scholar]
- 37.Levine R. L., Mosoni L., Berlett B. S., Stadtman E. R. Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. U.S.A. 1996;93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quijano C., Alvarez B., Gatti R. M., Augusto O., Radi R. Pathways of peroxynitrite oxidation of thiol groups. Biochem. J. 1997;322:167–173. doi: 10.1042/bj3220167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvarez B., Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids. 2003;25:295–311. doi: 10.1007/s00726-003-0018-8. [DOI] [PubMed] [Google Scholar]
- 40.Dux L., Taylor K. A., Ting-Beall H. P., Martonosi A. Crystallization of the Ca2+-ATPase of sarcoplasmic reticulum by calcium and lanthanide ions. J. Biol. Chem. 1985;260:11730–11743. [PubMed] [Google Scholar]