Abstract
An attempt was made to recover naturally processed T-cell determinants following antigen pulsing of tetanus toxin-specific human B-cell clones with microgram/ml amounts of antigen. Class II major histocompatibility complex (MHC) molecules were isolated from cells pulsed under optimal conditions and the eluted peptides displayed by reverse-phase high-performance liquid chromatography (HPLC). Antigen-pulsed and control-unpulsed cells showed virtually identical optical density (OD)215 profiles, although multiple peptides derived from the input antigen could be identified at the radiochemical level. At least four distinct HPLC fractions contained naturally processed versions of the determinant 830-844, detected using the specific T-cell clone Mix 111. Quantification of the most active fraction indicated that approximately 1 pmole of this determinant was recovered from approximately 5 x 10(9) antigen-pulsed cells. Based on the amount of antigen processed following uptake on membrane immunoglobulin, and quantification of the biologically active material recovered, it was estimated that the efficiency of determinant capture was no greater than 1-2%. Further method development and a considerable increase in the number of cells used (> 10(10)) would appear to be necessary before naturally processed determinants from exogenously pulsed antigens can be reliably and fully characterized. Finally, a theoretical analysis showed that accurate (+/- 0.01%) mass information alone could identify a limited number of candidate peptides from known or putative antigens.
Full text
PDF

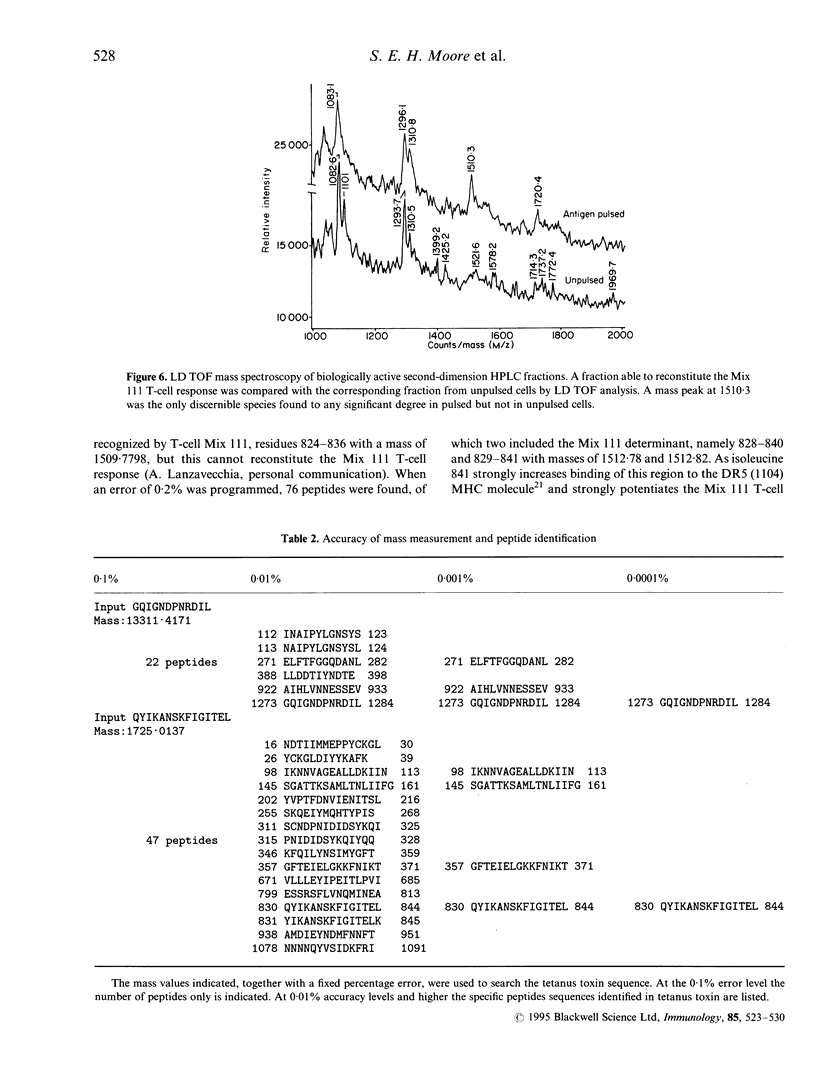


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amigorena S., Drake J. R., Webster P., Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 1994 May 12;369(6476):113–120. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- Davidson H. W., Reid P. A., Lanzavecchia A., Watts C. Processed antigen binds to newly synthesized MHC class II molecules in antigen-specific B lymphocytes. Cell. 1991 Oct 4;67(1):105–116. doi: 10.1016/0092-8674(91)90575-j. [DOI] [PubMed] [Google Scholar]
- Davidson H. W., Watts C. Epitope-directed processing of specific antigen by B lymphocytes. J Cell Biol. 1989 Jul;109(1):85–92. doi: 10.1083/jcb.109.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demotz S., Grey H. M., Appella E., Sette A. Characterization of a naturally processed MHC class II-restricted T-cell determinant of hen egg lysozyme. Nature. 1989 Dec 7;342(6250):682–684. doi: 10.1038/342682a0. [DOI] [PubMed] [Google Scholar]
- Guy K., Van Heyningen V., Cohen B. B., Deane D. L., Steel C. M. Differential expression and serologically distinct subpopulations of human Ia antigens detected with monoclonal antibodies to Ia alpha and beta chains. Eur J Immunol. 1982 Nov;12(11):942–948. doi: 10.1002/eji.1830121109. [DOI] [PubMed] [Google Scholar]
- Hammer J., Bono E., Gallazzi F., Belunis C., Nagy Z., Sinigaglia F. Precise prediction of major histocompatibility complex class II-peptide interaction based on peptide side chain scanning. J Exp Med. 1994 Dec 1;180(6):2353–2358. doi: 10.1084/jem.180.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. F., Michel H., Dickinson T. A., Shabanowitz J., Cox A. L., Sakaguchi K., Appella E., Grey H. M., Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992 Jun 26;256(5065):1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A., Reid P. A., Watts C. Irreversible association of peptides with class II MHC molecules in living cells. Nature. 1992 May 21;357(6375):249–252. doi: 10.1038/357249a0. [DOI] [PubMed] [Google Scholar]
- Nelson C. A., Roof R. W., McCourt D. W., Unanue E. R. Identification of the naturally processed form of hen egg white lysozyme bound to the murine major histocompatibility complex class II molecule I-Ak. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7380–7383. doi: 10.1073/pnas.89.16.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb J. R., Cresswell P. Characterization of endogenous peptides bound to purified HLA-DR molecules and their absence from invariant chain-associated alpha beta dimers. J Immunol. 1993 Jan 15;150(2):499–507. [PubMed] [Google Scholar]
- O'Sullivan D., Sidney J., Del Guercio M. F., Colón S. M., Sette A. Truncation analysis of several DR binding epitopes. J Immunol. 1991 Feb 15;146(4):1240–1246. [PubMed] [Google Scholar]
- Panina-Bordignon P., Tan A., Termijtelen A., Demotz S., Corradin G., Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989 Dec;19(12):2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., al-Ramadi B. K., Rothbard J., Janeway C. A., Jr Truncation variants of peptides isolated from MHC class II molecules suggest sequence motifs. Nature. 1992 Oct 1;359(6394):429–431. doi: 10.1038/359429a0. [DOI] [PubMed] [Google Scholar]
- Rudensky A., Janeway C. A., Jr Studies on naturally processed peptides associated with MHC class II molecules. Chem Immunol. 1993;57:134–151. [PubMed] [Google Scholar]
- Schneider C., Newman R. A., Sutherland D. R., Asser U., Greaves M. F. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem. 1982 Sep 25;257(18):10766–10769. [PubMed] [Google Scholar]
- Slingluff C. L., Jr, Cox A. L., Henderson R. A., Hunt D. F., Engelhard V. H. Recognition of human melanoma cells by HLA-A2.1-restricted cytotoxic T lymphocytes is mediated by at least six shared peptide epitopes. J Immunol. 1993 Apr 1;150(7):2955–2963. [PubMed] [Google Scholar]
- Tulp A., Verwoerd D., Dobberstein B., Ploegh H. L., Pieters J. Isolation and characterization of the intracellular MHC class II compartment. Nature. 1994 May 12;369(6476):120–126. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]
- Vignali D. A., Urban R. G., Chicz R. M., Strominger J. L. Minute quantities of a single immunodominant foreign epitope are presented as large nested sets by major histocompatibility complex class II molecules. Eur J Immunol. 1993 Jul;23(7):1602–1607. doi: 10.1002/eji.1830230731. [DOI] [PubMed] [Google Scholar]
- Watts C., Davidson H. W. Endocytosis and recycling of specific antigen by human B cell lines. EMBO J. 1988 Jul;7(7):1937–1945. doi: 10.1002/j.1460-2075.1988.tb03031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C., West M. A., Reid P. A., Davidson H. W. Processing of immunoglobulin-associated antigen in B lymphocytes. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):345–352. doi: 10.1101/sqb.1989.054.01.042. [DOI] [PubMed] [Google Scholar]
- West M. A., Lucocq J. M., Watts C. Antigen processing and class II MHC peptide-loading compartments in human B-lymphoblastoid cells. Nature. 1994 May 12;369(6476):147–151. doi: 10.1038/369147a0. [DOI] [PubMed] [Google Scholar]