Abstract
Resistance to Mycobacterium avium depends on both genetically encoded macrophage functions and acquired T-cell immunity. Cytokines may play a role in either type of resistance. We studied the expression of interleukin-2 (IL-2), IL-4 and interferon-gamma (IFN-gamma) in naturally susceptible BALB/c (Bcgs) and naturally resistant C.D2 (Bcgr) congenic mice infected with two strains of M. avium (one highly virulent and another of low virulence). We observed that cytokine expression patterns correlated better with the virulence of the micro-organism than with the genetic background of the host. The control of the infection by the low virulence strain in either mouse strain was associated with an increased expression of IFN-gamma and IL-2. Only Bcgs mice infected with a virulent strain of M. avium were unable to restrict bacterial growth. An increased expression of IL-4, early during infection, was detected in the course of the latter infection but played no role in determining the susceptibility to infection. Neutralization of IFN-gamma or IL-2 with specific monoclonal antibodies led to an exacerbation of the infection in Bcgr mice by the two strains of M. avium and in Bcgs mice infected with the low virulence strain of M. avium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg R., Castro A. G., Pedrosa J., Minóprio P. Role of interleukin-6 in the induction of protective T cells during mycobacterial infections in mice. Immunology. 1994 Jul;82(3):361–364. [PMC free article] [PubMed] [Google Scholar]
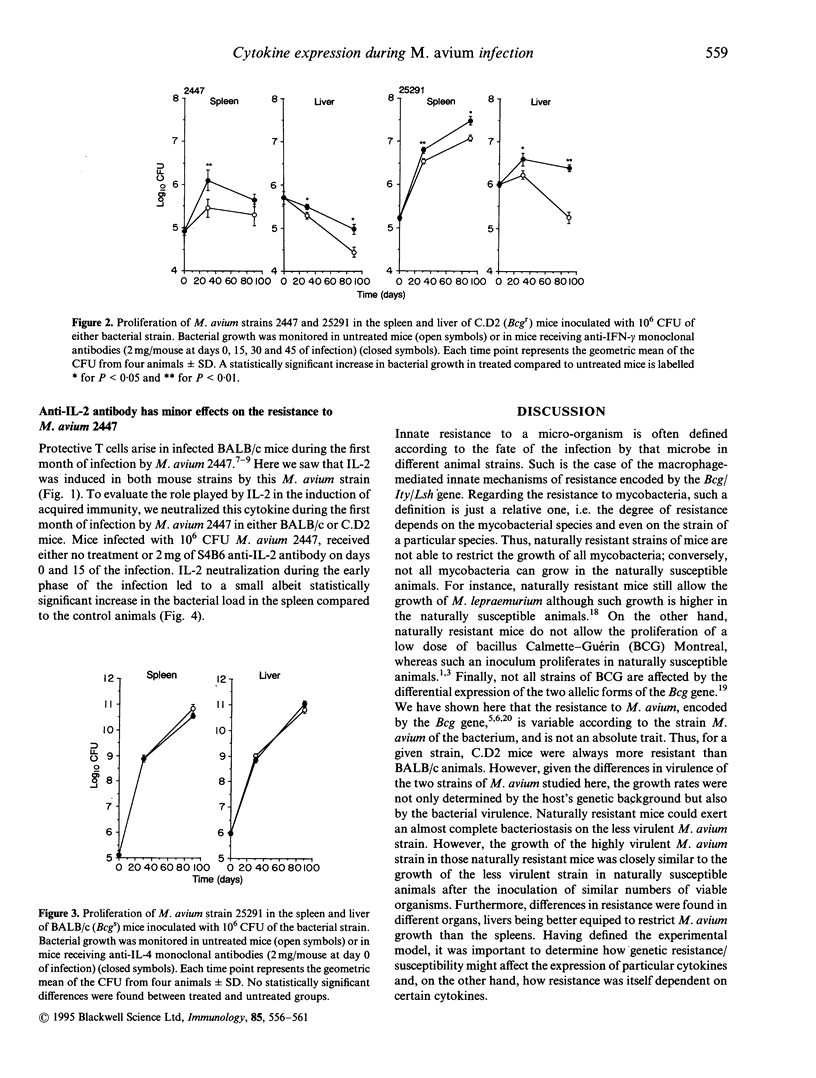
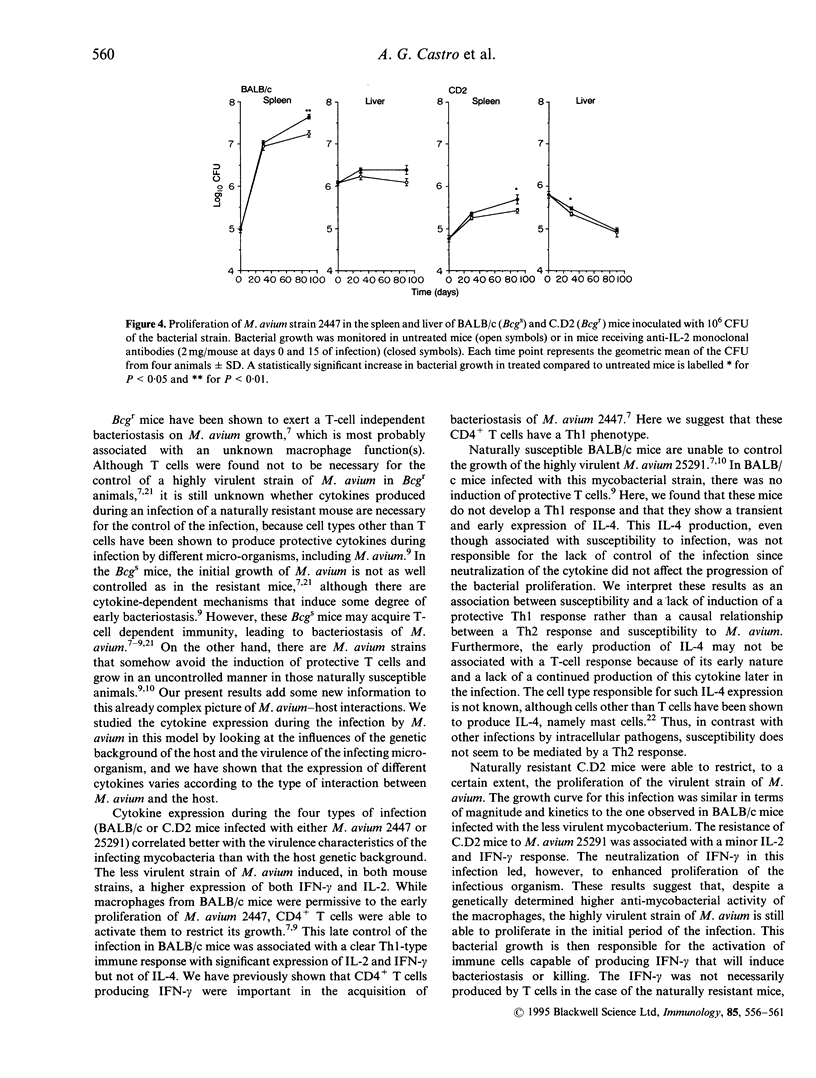
- Appelberg R., Castro A. G., Pedrosa J., Silva R. A., Orme I. M., Minóprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994 Sep;62(9):3962–3971. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Pedrosa J. Induction and expression of protective T cells during Mycobacterium avium infections in mice. Clin Exp Immunol. 1992 Mar;87(3):379–385. doi: 10.1111/j.1365-2249.1992.tb03006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Sarmento A. M. The role of macrophage activation and of Bcg-encoded macrophage function(s) in the control of Mycobacterium avium infection in mice. Clin Exp Immunol. 1990 Jun;80(3):324–331. doi: 10.1111/j.1365-2249.1990.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown I. N., Glynn A. A., Plant J. Inbred mouse strain resistance to Mycobacterium lepraemurium follows the Ity/Lsh pattern. Immunology. 1982 Sep;47(1):149–156. [PMC free article] [PubMed] [Google Scholar]
- Buschman E., Apt A. S., Nickonenko B. V., Moroz A. M., Averbakh M. H., Skamene E. Genetic aspects of innate resistance and acquired immunity to mycobacteria in inbred mice. Springer Semin Immunopathol. 1988;10(4):319–336. doi: 10.1007/BF02053844. [DOI] [PubMed] [Google Scholar]
- Goto Y., Buschman E., Skamene E. Regulation of host resistance to Mycobacterium intracellulare in vivo and in vitro by the Bcg gene. Immunogenetics. 1989;30(3):218–221. doi: 10.1007/BF02421210. [DOI] [PubMed] [Google Scholar]
- Minoprio P., el Cheikh M. C., Murphy E., Hontebeyrie-Joskowicz M., Coffman R., Coutinho A., O'Garra A. Xid-associated resistance to experimental Chagas' disease is IFN-gamma dependent. J Immunol. 1993 Oct 15;151(8):4200–4208. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Street N. F., Budd R., O'Garra A., Fong T. A., Bond M. W., Moore K. W., Sher A., Fiorentino D. F. Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol Rev. 1991 Oct;123:209–229. doi: 10.1111/j.1600-065x.1991.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Murphy E., Hieny S., Sher A., O'Garra A. Detection of in vivo expression of interleukin-10 using a semi-quantitative polymerase chain reaction method in Schistosoma mansoni infected mice. J Immunol Methods. 1993 Jun 18;162(2):211–223. doi: 10.1016/0022-1759(93)90386-l. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Roberts A. D., Griffin J. P., Abrams J. S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993 Jul 1;151(1):518–525. [PubMed] [Google Scholar]
- Orme I. M., Stokes R. W., Collins F. M. Genetic control of natural resistance to nontuberculous mycobacterial infections in mice. Infect Immun. 1986 Oct;54(1):56–62. doi: 10.1128/iai.54.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E., Seder R. A., Plaut M. Lymphokine and cytokine production by Fc epsilon RI+ cells. Adv Immunol. 1993;53:1–29. [PubMed] [Google Scholar]
- Pedrosa J., Flórido M., Kunze Z. M., Castro A. G., Portaels F., McFadden J., Silva M. T., Appelberg R. Characterization of the virulence of Mycobacterium avium complex (MAC) isolates in mice. Clin Exp Immunol. 1994 Nov;98(2):210–216. doi: 10.1111/j.1365-2249.1994.tb06127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., O'Brien A. D., Skamene E., Gros P., Forget A., Kongshavn P. A., Wax J. S. A BALB/c congenic strain of mice that carries a genetic locus (Ityr) controlling resistance to intracellular parasites. Infect Immun. 1983 Jun;40(3):1234–1235. doi: 10.1128/iai.40.3.1234-1235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramarathinam L., Niesel D. W., Klimpel G. R. Ity influences the production of IFN-gamma by murine splenocytes stimulated in vitro with Salmonella typhimurium. J Immunol. 1993 May 1;150(9):3965–3972. [PubMed] [Google Scholar]
- Ramarathinam L., Niesel D. W., Klimpel G. R. Salmonella typhimurium induces IFN-gamma production in murine splenocytes. Role of natural killer cells and macrophages. J Immunol. 1993 May 1;150(9):3973–3981. [PubMed] [Google Scholar]
- Reed S. G., Scott P. T-cell and cytokine responses in leishmaniasis. Curr Opin Immunol. 1993 Aug;5(4):524–531. doi: 10.1016/0952-7915(93)90033-o. [DOI] [PubMed] [Google Scholar]
- Stokes R. W., Collins F. M. Passive transfer of immunity of Mycobacterium avium in susceptible and resistant strains of mice. Clin Exp Immunol. 1990 Jul;81(1):109–115. doi: 10.1111/j.1365-2249.1990.tb05299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S. M., Malo D., Vogan K., Skamene E., Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993 May 7;73(3):469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]


