Abstract
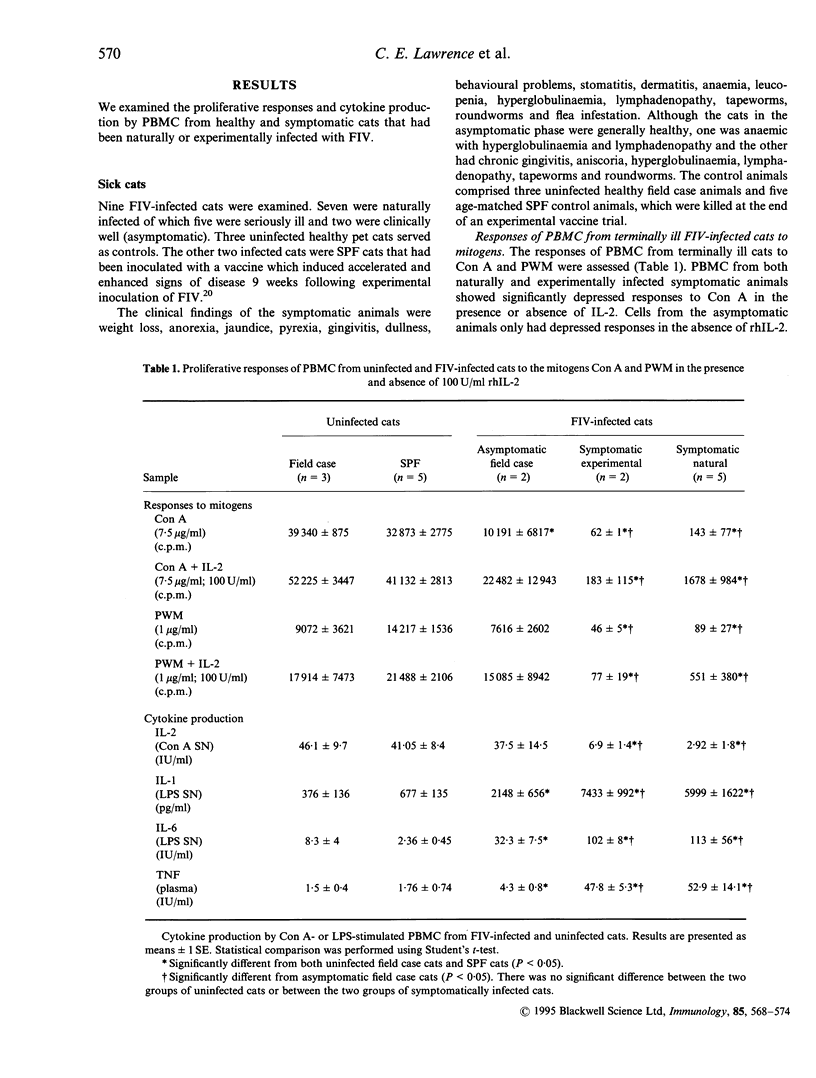
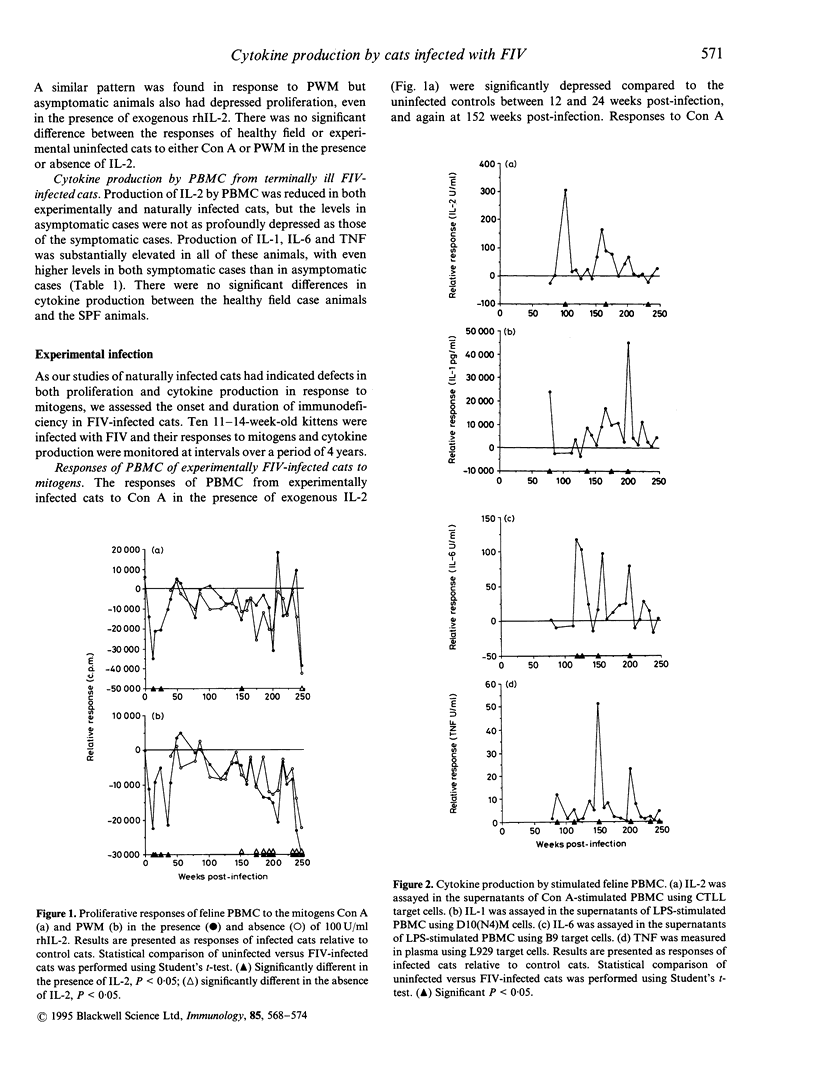
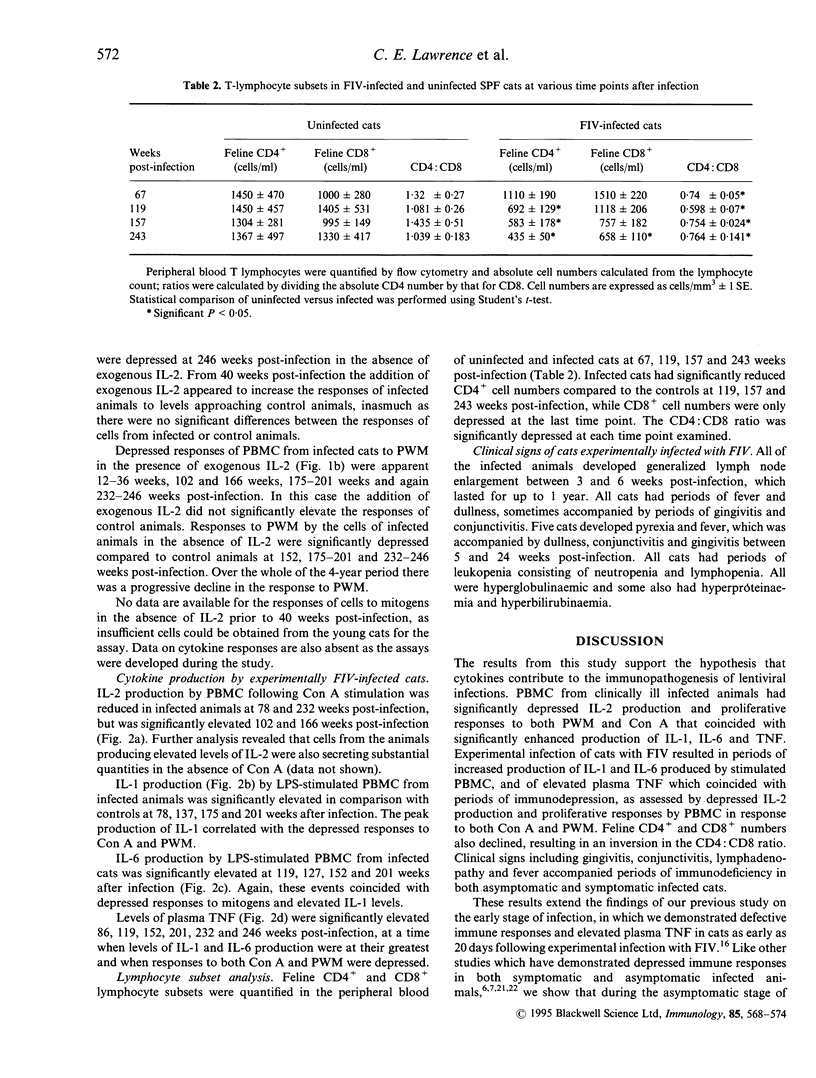
The immune responsiveness of cats naturally or experimentally infected with feline immunodeficiency virus (FIV) was studied. Peripheral blood mononuclear cells (PBMC) from naturally infected, symptomatic animals displayed depressed proliferation and interleukin-2 (IL-2) production in response to mitogens, which was accompanied by a significant increase in IL-1, IL-6 and tumour necrosis factor (TNF) production. Longitudinal studies were performed over a period of 4 years in experimentally infected animals. The responses of cells from these cats to concanavalin A (Con A) were consistently less than those from uninfected cats throughout the period but, owing to variation between cats, were significantly lower on only a few occasions. By contrast, the responses of cells to pokeweed mitogen (PWM) were severely affected and declined progressively throughout the 4-year period. In general, responses to Con A but not PWM could be restored by the addition of exogenous IL-2. The decline in immune responsiveness was concurrent with a decline in feline (f)CD4+ cells and an inversion in the CD4:CD8 ratio. Peak production of IL-1, IL-6 and TNF coincided with periods of depressed immune responses. Additionally, immunodeficient responses and elevated levels of proinflammatory cytokines were concurrent with the presence of clinical signs. We conclude that, like human immunodeficiency virus (HIV), FIV infection results in significant perturbation of the immune response. Responses to PWM appear to correlate with disease progression which suggests that the CD3 pathway is affected in the earlier stages of the disease and that additional activation pathways such as CD2 may not be affected until the animal enters the acquired immune deficient syndrome (AIDS) stage of the disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Ackley C. D., Yamamoto J. K., Levy N., Pedersen N. C., Cooper M. D. Immunologic abnormalities in pathogen-free cats experimentally infected with feline immunodeficiency virus. J Virol. 1990 Nov;64(11):5652–5655. doi: 10.1128/jvi.64.11.5652-5655.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann A. J., Abrams D., Conant M., Chudwin D., Cowan M., Volberding P., Lewis B., Casavant C. Acquired immune dysfunction in homosexual men: immunologic profiles. Clin Immunol Immunopathol. 1983 Jun;27(3):315–325. doi: 10.1016/0090-1229(83)90084-3. [DOI] [PubMed] [Google Scholar]
- Beebe A. M., Dua N., Faith T. G., Moore P. F., Pedersen N. C., Dandekar S. Primary stage of feline immunodeficiency virus infection: viral dissemination and cellular targets. J Virol. 1994 May;68(5):3080–3091. doi: 10.1128/jvi.68.5.3080-3091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callanan J. J., McCandlish I. A., O'Neil B., Lawrence C. E., Rigby M., Pacitti A. M., Jarrett O. Lymphosarcoma in experimentally induced feline immunodeficiency virus infection [corrected]. Vet Rec. 1992 Apr 4;130(14):293–295. doi: 10.1136/vr.130.14.293. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Lucey D. R., Berzofsky J. A., Pinto L. A., Wynn T. A., Blatt S. P., Dolan M. J., Hendrix C. W., Wolf S. F., Shearer G. M. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science. 1993 Dec 10;262(5140):1721–1724. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- Clerici M., Shearer G. M. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993 Mar;14(3):107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- Flynn J. N., Cannon C. A., Lawrence C. E., Jarrett O. Polyclonal B-cell activation in cats infected with feline immunodeficiency virus. Immunology. 1994 Apr;81(4):626–630. [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Hoffmann-Fezer G., Thum J., Ackley C., Herbold M., Mysliwietz J., Thefeld S., Hartmann K., Kraft W. Decline in CD4+ cell numbers in cats with naturally acquired feline immunodeficiency virus infection. J Virol. 1992 Mar;66(3):1484–1488. doi: 10.1128/jvi.66.3.1484-1488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann B., Jakobsen K. D., Odum N., Dickmeiss E., Platz P., Ryder L. P., Pedersen C., Mathiesen L., Bygbjerg I. B., Faber V. HIV-induced immunodeficiency. Relatively preserved phytohemagglutinin as opposed to decreased pokeweed mitogen responses may be due to possibly preserved responses via CD2/phytohemagglutinin pathway. J Immunol. 1989 Mar 15;142(6):1874–1880. [PubMed] [Google Scholar]
- Hopkins S. J., Humphreys M. Simple, sensitive and specific bioassay of interleukin-1. J Immunol Methods. 1989 Jun 21;120(2):271–276. doi: 10.1016/0022-1759(89)90252-4. [DOI] [PubMed] [Google Scholar]
- Hosie M. J., Jarrett O. Serological responses of cats to feline immunodeficiency virus. AIDS. 1990 Mar;4(3):215–220. doi: 10.1097/00002030-199003000-00006. [DOI] [PubMed] [Google Scholar]
- Hosie M. J., Osborne R., Reid G., Neil J. C., Jarrett O. Enhancement after feline immunodeficiency virus vaccination. Vet Immunol Immunopathol. 1992 Dec;35(1-2):191–197. doi: 10.1016/0165-2427(93)90149-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. E., Callanan J. J., Jarrett O. Decreased mitogen responsiveness and elevated tumor necrosis factor production in cats shortly after feline immunodeficiency virus infection. Vet Immunol Immunopathol. 1992 Dec;35(1-2):51–59. doi: 10.1016/0165-2427(92)90120-f. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Kobayashi N., Yamamoto N. Cytokines and HIV infection: is AIDS a tumor necrosis factor disease? AIDS. 1991 Dec;5(12):1405–1417. doi: 10.1097/00002030-199112000-00001. [DOI] [PubMed] [Google Scholar]
- Moore K. W., O'Garra A., de Waal Malefyt R., Vieira P., Mosmann T. R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Ohashi T., Goitsuka R., Watari T., Tsujimoto H., Hasegawa A. Elevation of feline interleukin 6-like activity in feline immunodeficiency virus infection. Clin Immunol Immunopathol. 1992 Dec;65(3):207–211. doi: 10.1016/0090-1229(92)90148-h. [DOI] [PubMed] [Google Scholar]
- Pauza C. D. HIV persistence in monocytes leads to pathogenesis and AIDS. Cell Immunol. 1988 Apr 1;112(2):414–424. doi: 10.1016/0008-8749(88)90310-3. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Ho E. W., Brown M. L., Yamamoto J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987 Feb 13;235(4790):790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Shelton G. H., Linenberger M. L., Grant C. K., Abkowitz J. L. Hematologic manifestations of feline immunodeficiency virus infection. Blood. 1990 Sep 15;76(6):1104–1109. [PubMed] [Google Scholar]
- Siebelink K. H., Chu I. H., Rimmelzwaan G. F., Weijer K., van Herwijnen R., Knell P., Egberink H. F., Bosch M. L., Osterhaus A. D. Feline immunodeficiency virus (FIV) infection in the cat as a model for HIV infection in man: FIV-induced impairment of immune function. AIDS Res Hum Retroviruses. 1990 Dec;6(12):1373–1378. doi: 10.1089/aid.1990.6.1373. [DOI] [PubMed] [Google Scholar]
- Sinicco A., Biglino A., Sciandra M., Forno B., Pollono A. M., Raiteri R., Gioannini P. Cytokine network and acute primary HIV-1 infection. AIDS. 1993 Sep;7(9):1167–1172. doi: 10.1097/00002030-199309000-00003. [DOI] [PubMed] [Google Scholar]
- Tindall B., Cooper D. A. Primary HIV infection: host responses and intervention strategies. AIDS. 1991 Jan;5(1):1–14. [PubMed] [Google Scholar]
- Torten M., Franchini M., Barlough J. E., George J. W., Mozes E., Lutz H., Pedersen N. C. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J Virol. 1991 May;65(5):2225–2230. doi: 10.1128/jvi.65.5.2225-2230.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyakarnam A., McKeating J., Meager A., Beverley P. C. Tumour necrosis factors (alpha, beta) induced by HIV-1 in peripheral blood mononuclear cells potentiate virus replication. AIDS. 1990 Jan;4(1):21–27. doi: 10.1097/00002030-199001000-00003. [DOI] [PubMed] [Google Scholar]
- Willett B. J., Hosie M. J., Callanan J. J., Neil J. C., Jarrett O. Infection with feline immunodeficiency virus is followed by the rapid expansion of a CD8+ lymphocyte subset. Immunology. 1993 Jan;78(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J. K., Hansen H., Ho E. W., Morishita T. Y., Okuda T., Sawa T. R., Nakamura R. M., Pedersen N. C. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J Am Vet Med Assoc. 1989 Jan 15;194(2):213–220. [PubMed] [Google Scholar]


