Abstract
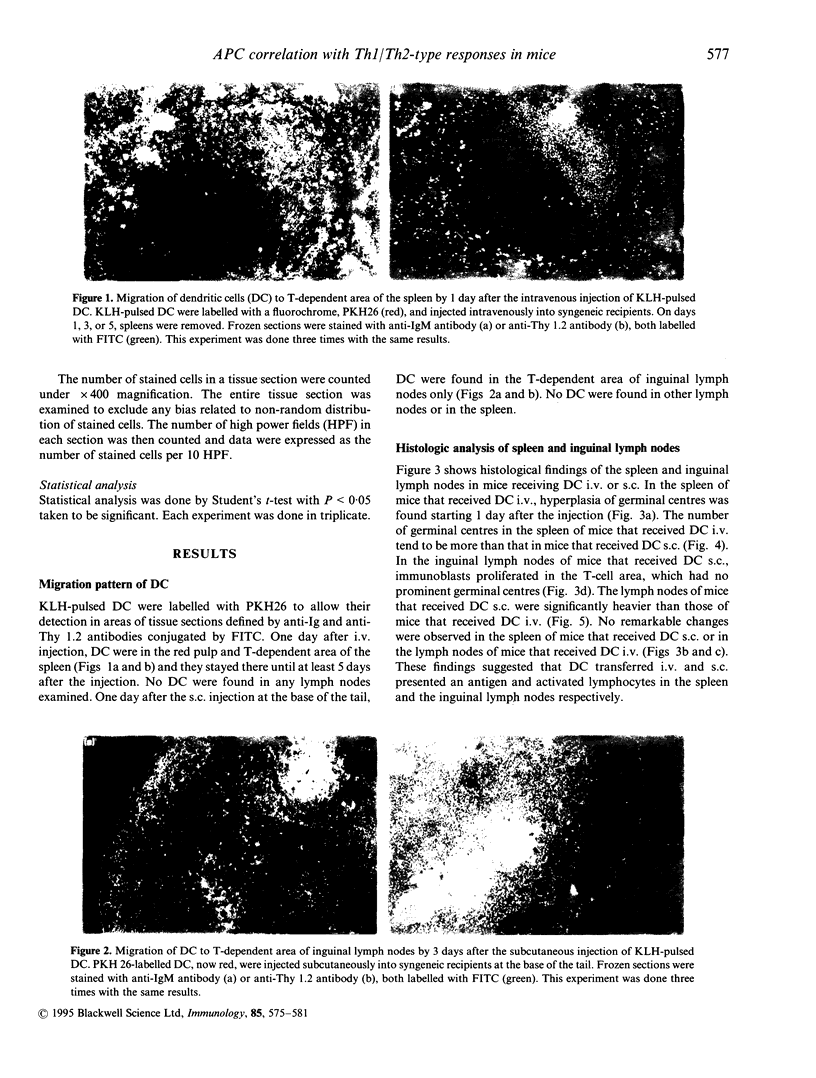
Antibodies are produced when antigen-presenting cells (APC) pulsed with an antigen are injected intravenously (i.v.) into BALB/c mice, but subcutaneous (s.c.) injection of such APC causes delayed-type hypersensitivity (DTH). To identify the anatomic sites where T and B cells are activated, we labelled splenic dendritic cells (DC) with a fluorochrome, PKH 26, injected them i.v. or s.c., and used the label to locate them. When the DC were injected i.v., germinal centres in the spleen were hyperplastic on day 1. Most DC moved to T-dependent areas of the white and red pulp on day 1 and remained there at least until day 5, but no DC migrated into the lymph nodes. When the DC were injected s.c., they were in the sinus on day 1 and had entered T-dependent area of draining lymph nodes only by day 3; hyperplasia of germinal centres in the spleen and migration of DC into the spleen were not found. We used the polymerase chain reaction (PCR) to study which mice had spleen cells and lymph node cells that produced the cytokines interleukin (IL)-2, IL-4, IL-10, and interferon-gamma (IFN-gamma). In the sensitization phase, day 1 after DC injection i.v., almost all IL-10 transcript was found in spleen cells, but after DC injection s.c., IL-2 message was most abundant in lymph node cells. The expression of mRNA for IL-4 and IFN-gamma in mice that received DC i.v. was not different from that in mice that received DC s.c. in this phase. Immunohistochemical staining showed that cells stained for IL-10 were in the T-dependent area of the spleen from mice that received DC i.v. 1 day after the injection. Three days after the injection of DC i.v., cells stained for IL-10 were in the germinal centres as well. The number of such cells in the spleen of mice that received DC i.v. was significantly more than that in mice that received DC s.c. IL-10 may be important in development of TH2 response.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Kupiec-Weglinski J. W., Hankins D. F., Morris P. J. Migration patterns of dendritic cells in the mouse. Homing to T cell-dependent areas of spleen, and binding within marginal zone. J Exp Med. 1988 Feb 1;167(2):646–651. doi: 10.1084/jem.167.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Modlin R. L., Salgame P. Stigma variations: observations on suppressor T cells and leprosy. Annu Rev Immunol. 1992;10:453–488. doi: 10.1146/annurev.iy.10.040192.002321. [DOI] [PubMed] [Google Scholar]
- Boom W. H., Liano D., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. II. Effects of interleukin 4- and interleukin 2-producing T cell clones on resting B lymphocytes. J Exp Med. 1988 Apr 1;167(4):1350–1363. doi: 10.1084/jem.167.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. L., Shea C. M., Urioste S., Thompson R. C., Boom W. H., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. III. Responses of IL-2- and IL-4-producing (Th1 and Th2) clones to antigens presented by different accessory cells. J Immunol. 1990 Nov 1;145(9):2803–2808. [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Varkila K., Scott P., Chatelain R. Role of cytokines in the differentiation of CD4+ T-cell subsets in vivo. Immunol Rev. 1991 Oct;123:189–207. doi: 10.1111/j.1600-065x.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- DeKruyff R. H., Fang Y., Umetsu D. T. IL-4 synthesis by in vivo primed keyhole limpet hemocyanin-specific CD4+ T cells. I. Influence of antigen concentration and antigen-presenting cell type. J Immunol. 1992 Dec 1;149(11):3468–3476. [PubMed] [Google Scholar]
- Fernandez-Botran R., Sanders V. M., Mosmann T. R., Vitetta E. S. Lymphokine-mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J Exp Med. 1988 Aug 1;168(2):543–558. doi: 10.1084/jem.168.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Flores Villanueva P. O., Chikunguwo S. M., Harris T. S., Stadecker M. J. Role of IL-10 on antigen-presenting cell function for schistosomal egg-specific monoclonal T helper cell responses in vitro and in vivo. J Immunol. 1993 Sep 15;151(6):3192–3198. [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Differential activation of murine TH1 and TH2 clones. Res Immunol. 1991 Jan;142(1):19–23. doi: 10.1016/0923-2494(91)90005-4. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Schell S. R., Nau G., Fitch F. W. Regulation of T-cell activation: differences among T-cell subsets. Immunol Rev. 1989 Oct;111:79–110. doi: 10.1111/j.1600-065x.1989.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Greene M. I., Sugimoto M., Benacerraf B. Mechanisms of regulation of cell-mediated immune responses. I. Effect of the route of immunization with TNP-coupled syngeneic cells on the induction and suppression of contact sensitivity to picryl chloride. J Immunol. 1978 May;120(5):1604–1611. [PubMed] [Google Scholar]
- Heath A. W., Playfair J. H. Conjugation of interferon-gamma to antigen enhances its adjuvanticity. Immunology. 1990 Nov;71(3):454–456. [PMC free article] [PubMed] [Google Scholar]
- Ho A. S., Moore K. W. Interleukin-10 and its receptor. Ther Immunol. 1994 Jun;1(3):173–185. [PubMed] [Google Scholar]
- Howard M., O'Garra A., Ishida H., de Waal Malefyt R., de Vries J. Biological properties of interleukin 10. J Clin Immunol. 1992 Jul;12(4):239–247. doi: 10.1007/BF00918147. [DOI] [PubMed] [Google Scholar]
- Katsura Y. Cell-mediated and humoral immune responses in mice. III. Dynamic balance between delayed-type hypersensitivity and antibody response. Immunology. 1977 Mar;32(3):227–235. [PMC free article] [PubMed] [Google Scholar]
- Killar L., MacDonald G., West J., Woods A., Bottomly K. Cloned, Ia-restricted T cells that do not produce interleukin 4(IL 4)/B cell stimulatory factor 1(BSF-1) fail to help antigen-specific B cells. J Immunol. 1987 Mar 15;138(6):1674–1679. [PubMed] [Google Scholar]
- Krug M. S., Berger S. L. First-strand cDNA synthesis primed with oligo(dT). Methods Enzymol. 1987;152:316–325. doi: 10.1016/0076-6879(87)52036-5. [DOI] [PubMed] [Google Scholar]
- Macatonia S. E., Doherty T. M., Knight S. C., O'Garra A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol. 1993 May 1;150(9):3755–3765. [PubMed] [Google Scholar]
- Macon W. R., Cousar J. B., Waldron J. A., Jr, Hsu S. M. Interleukin-4 may contribute to the abundant T-cell reaction and paucity of neoplastic B cells in T-cell-rich B-cell lymphomas. Am J Pathol. 1992 Nov;141(5):1031–1036. [PMC free article] [PubMed] [Google Scholar]
- Morikawa Y., Furotani M., Kuribayashi K., Matsuura N., Kakudo K. The role of antigen-presenting cells in the regulation of delayed-type hypersensitivity. I. Spleen dendritic cells. Immunology. 1992 Sep;77(1):81–87. [PMC free article] [PubMed] [Google Scholar]
- Morikawa Y., Furotani M., Matsuura N., Kakudo K. The role of antigen-presenting cells in the regulation of delayed-type hypersensitivity. II. Epidermal Langerhans' cells and peritoneal exudate macrophages. Cell Immunol. 1993 Nov;152(1):200–210. doi: 10.1006/cimm.1993.1279. [DOI] [PubMed] [Google Scholar]
- Morikawa Y., Kuribayashi K., Saito K. B-cell-mediated regulation of delayed-type hypersensitivity. Cell Immunol. 1990 Dec;131(2):338–351. doi: 10.1016/0008-8749(90)90259-t. [DOI] [PubMed] [Google Scholar]
- Morikawa Y., Kuribayashi K., Saito K. Immunoregulatory effect of antibody on delayed-type hypersensitivity in mice. Int Arch Allergy Appl Immunol. 1989;90(2):130–136. doi: 10.1159/000235014. [DOI] [PubMed] [Google Scholar]
- Morikawa Y., Kuribayashi K., Yoshikawa F., Fujita K., Mizushima A., Kakudo K. The role of antibodies in the regulation of delayed-type hypersensitivity. Immunology. 1991 Sep;74(1):146–152. [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Parish C. R. The relationship between humoral and cell-mediated immunity. Transplant Rev. 1972;13:35–66. doi: 10.1111/j.1600-065x.1972.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Stout R. D., Bottomly K. Antigen-specific activation of effector macrophages by IFN-gamma producing (TH1) T cell clones. Failure of IL-4-producing (TH2) T cell clones to activate effector function in macrophages. J Immunol. 1989 Feb 1;142(3):760–765. [PubMed] [Google Scholar]
- Sugimura K., Kishimoto T., Maeda K., Yamamura Y. Demonstration of T15 idiotype-positive effector and suppressor T cells for phosphorylcholine-specific delayed-type hypersensitivity response in CBA/N or (CBA/N X BALB/c)F1 male mice. Eur J Immunol. 1981 Jun;11(6):455–461. doi: 10.1002/eji.1830110604. [DOI] [PubMed] [Google Scholar]
- Yang X., Gieni R. S., Mosmann T. R., HayGlass K. T. Chemically modified antigen preferentially elicits induction of Th1-like cytokine synthesis patterns in vivo. J Exp Med. 1993 Jul 1;178(1):349–353. doi: 10.1084/jem.178.1.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A., Moore K. W. Interleukin 10. Cytokine. 1991 Sep;3(5):366–371. doi: 10.1016/1043-4666(91)90039-g. [DOI] [PubMed] [Google Scholar]







