Abstract
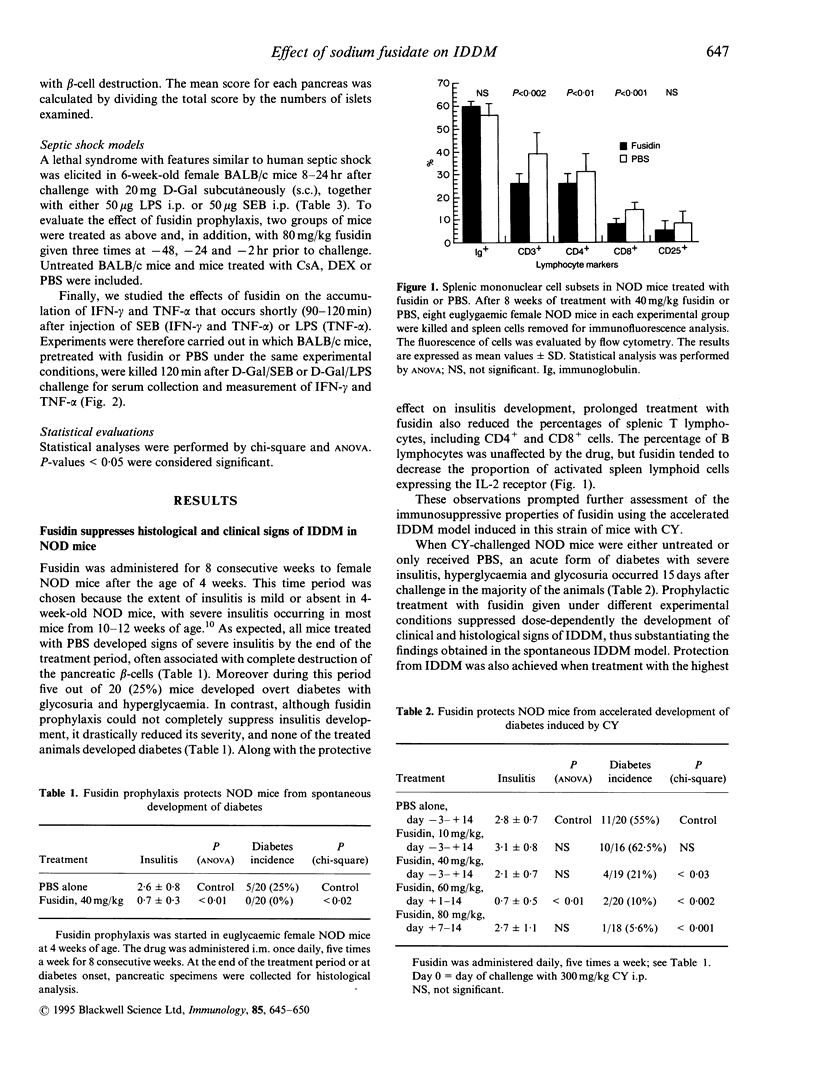
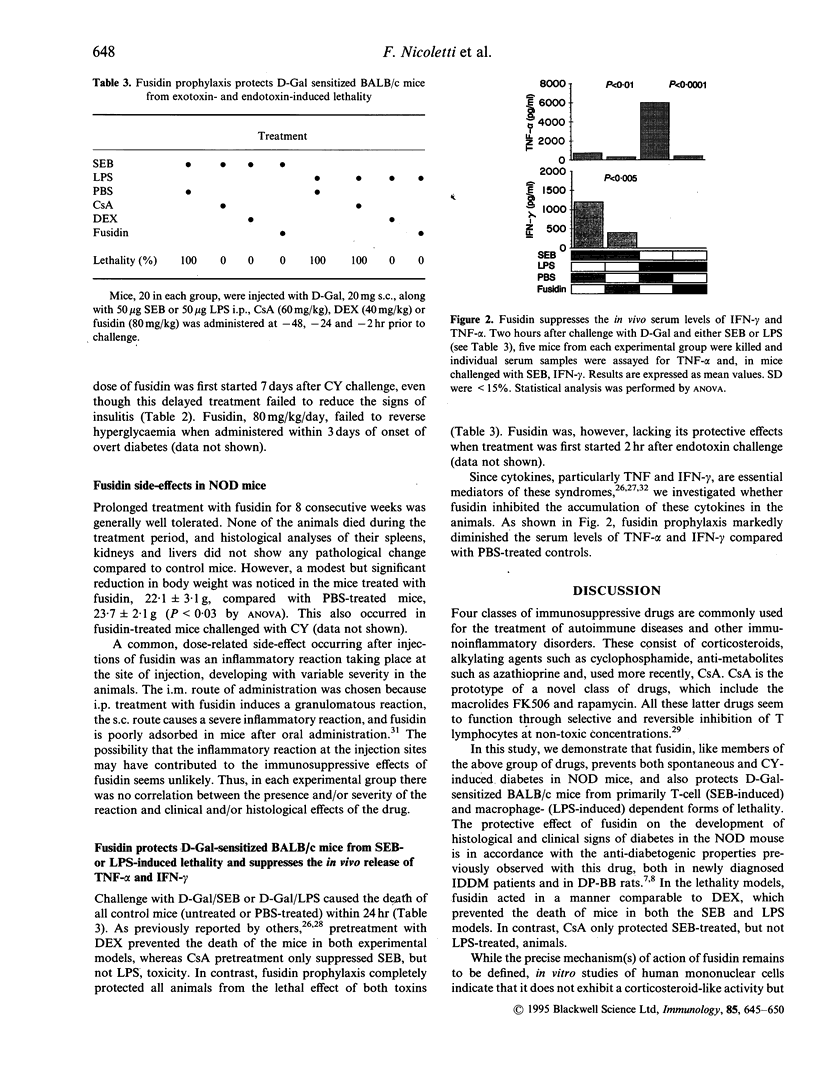
We have evaluated the effects of the novel immunosuppressant sodium fusidate (fusidin) in the non-obese diabetic (NOD) mouse and in D-galactosamine (D-Gal)-presensitized BALB/c mice challenged with the bacterial superantigen, Staphylococcus aureus enterotoxin B (SEB) or with the endotoxin, Escherichia coli lipopolysaccharide (LPS). The NOD mouse model has clinical and histoimmunological features similar to those of human insulin-dependent diabetes mellitus (IDDM). The SEB- and LPS-treated BALB/c mouse models exhibit pathogenic similarities with human septic shock conditions. In the NOD mouse, fusidin suppressed the spontaneous development of insulitis (mean inhibition 73%) and hyperglycaemia (IDDM incidence 25% versus 0%) when administered at 40 mg/kg five times weekly for 8 consecutive weeks from the fourth week of age; concurrently treated animals exhibited reduced percentages of splenic T lymphocytes. This anti-diabetogenic effect was confirmed in the accelerated model of diabetes induced in the NOD mouse with cyclophosphamide (CY) (IDDM incidence 55% versus 21-6% using dosages of fusidin from 40 to 80 mg/kg five times weekly); protection from IDDM development was achieved even when the drug (80 mg/kg/day) was first administered 7 days after CY challenge. In contrast, fusidin did not reverse hyperglycaemia when administered to CY-treated animals within 3 days of IDDM development. In the two models of septic shock, prophylactic treatment with fusidin, 80 mg/kg given three times for 2 days prior to D-Gal/SEB or D-Gal/LPS challenge, drastically reduced the lethality compared with D-Gal/buffer-treated mice. This effect may depend on the inhibitory action of fusidin on the secretion of cytokines such as interferon-gamma and tumour necrosis factor-alpha, the serum levels of which were greatly diminished in the fusidin-treated mice (mean inhibition 50-90%). These results demonstrate that fusidin may have a role in the treatment of cell-mediated autoimmune diseases and cytokine-mediated infectious diseases in humans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assan R., Feutren G., Debray-Sachs M., Quiniou-Debrie M. C., Laborie C., Thomas G., Chatenoud L., Bach J. F. Metabolic and immunological effects of cyclosporin in recently diagnosed type 1 diabetes mellitus. Lancet. 1985 Jan 12;1(8420):67–71. doi: 10.1016/s0140-6736(85)91964-6. [DOI] [PubMed] [Google Scholar]
- Bacelj A., Charlton B., Mandel T. E. Prevention of cyclophosphamide-induced diabetes by anti-V beta 8 T-lymphocyte-receptor monoclonal antibody therapy in NOD/Wehi mice. Diabetes. 1989 Nov;38(11):1492–1495. doi: 10.2337/diab.38.11.1492. [DOI] [PubMed] [Google Scholar]
- Baeder W. L., Sredy J., Sehgal S. N., Chang J. Y., Adams L. M. Rapamycin prevents the onset of insulin-dependent diabetes mellitus (IDDM) in NOD mice. Clin Exp Immunol. 1992 Aug;89(2):174–178. doi: 10.1111/j.1365-2249.1992.tb06928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtzen K., Diamant M., Faber V. Fusidic acid, an immunosuppressive drug with functions similar to cyclosporin A. Cytokine. 1990 Nov;2(6):423–429. doi: 10.1016/1043-4666(90)90051-t. [DOI] [PubMed] [Google Scholar]
- Bendtzen K., Diamant M., Horn T., Pedersen C., Buschard K. Effect of fusidic acid on interleukin-1 (IL-1)- and IL-6-induced pancreatic beta-cell functions in rats. J Endocrinol. 1992 Mar;132(3):345–352. doi: 10.1677/joe.0.1320345. [DOI] [PubMed] [Google Scholar]
- Bendtzen K. Immune hormones (cytokines); pathogenic role in autoimmune rheumatic and endocrine diseases. Autoimmunity. 1989;2(2):177–189. doi: 10.3109/08916938909019954. [DOI] [PubMed] [Google Scholar]
- Bendtzen K., Vesti-Nielsen N., Petersen J., Andersen V., Bendixen G. Treatment of chronic endogenous uveitis with fusidic acid. Lancet. 1991 Mar 2;337(8740):552–553. doi: 10.1016/0140-6736(91)91337-t. [DOI] [PubMed] [Google Scholar]
- Campbell I. L., Kay T. W., Oxbrow L., Harrison L. C. Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J Clin Invest. 1991 Feb;87(2):739–742. doi: 10.1172/JCI115055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll P. B., Strasser S., Alejandro R. The effect of FK 506 on cyclophosphamide-induced diabetes in the NOD mouse model. Transplant Proc. 1991 Dec;23(6):3348–3350. [PubMed] [Google Scholar]
- Castaño L., Eisenbarth G. S. Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat. Annu Rev Immunol. 1990;8:647–679. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- Charlton B., Bacelj A., Mandel T. E. Administration of silica particles or anti-Lyt2 antibody prevents beta-cell destruction in NOD mice given cyclophosphamide. Diabetes. 1988 Jul;37(7):930–935. doi: 10.2337/diab.37.7.930. [DOI] [PubMed] [Google Scholar]
- Charlton B., Bacelj A., Slattery R. M., Mandel T. E. Cyclophosphamide-induced diabetes in NOD/WEHI mice. Evidence for suppression in spontaneous autoimmune diabetes mellitus. Diabetes. 1989 Apr;38(4):441–447. doi: 10.2337/diab.38.4.441. [DOI] [PubMed] [Google Scholar]
- Debray-Sachs M., Carnaud C., Boitard C., Cohen H., Gresser I., Bedossa P., Bach J. F. Prevention of diabetes in NOD mice treated with antibody to murine IFN gamma. J Autoimmun. 1991 Apr;4(2):237–248. doi: 10.1016/0896-8411(91)90021-4. [DOI] [PubMed] [Google Scholar]
- Dupré J., Stiller C. R., Gent M., Donner A., von Graffenreid B., Murphy G., Heinrichs D., Jenner M. R., Keown P. A., Laupacis A. Effects of immunosuppression with cyclosporine in insulin-dependent diabetes mellitus of recent onset: the Canadian open study at 44 months. Transplant Proc. 1988 Jun;20(3 Suppl 4):184–192. [PubMed] [Google Scholar]
- Feutren G., Papoz L., Assan R., Vialettes B., Karsenty G., Vexiau P., Du Rostu H., Rodier M., Sirmai J., Lallemand A. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet. 1986 Jul 19;2(8499):119–124. doi: 10.1016/s0140-6736(86)91943-4. [DOI] [PubMed] [Google Scholar]
- Findon G., Miller T. E., Rowe L. C. Pharmacokinetics of fusidic acid in laboratory animals. Lab Anim Sci. 1991 Oct;41(5):462–465. [PubMed] [Google Scholar]
- GODTFREDSEN W. O., JAHNSEN S., LORCK H., ROHOLT K., TYBRING L. Fusidic acid: a new antibiotic. Nature. 1962 Mar 10;193:987–987. doi: 10.1038/193987a0. [DOI] [PubMed] [Google Scholar]
- Gonzalo J. A., González-García A., Kalland T., Hedlund G., Martínez C., Kroemer G. Linomide, a novel immunomodulator that prevents death in four models of septic shock. Eur J Immunol. 1993 Sep;23(9):2372–2374. doi: 10.1002/eji.1830230949. [DOI] [PubMed] [Google Scholar]
- Han J., Thompson P., Beutler B. Dexamethasone and pentoxifylline inhibit endotoxin-induced cachectin/tumor necrosis factor synthesis at separate points in the signaling pathway. J Exp Med. 1990 Jul 1;172(1):391–394. doi: 10.1084/jem.172.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M., Makino S. Promotion of spontaneous diabetes in non-obese diabetes-prone mice by cyclophosphamide. Diabetologia. 1984 Dec;27(6):604–606. doi: 10.1007/BF00276978. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P. The role of IFN-gamma in the pathology of experimental endotoxemia. J Immunol. 1990 Nov 1;145(9):2920–2924. [PubMed] [Google Scholar]
- Lampeter E. F., Signore A., Gale E. A., Pozzilli P. Lessons from the NOD mouse for the pathogenesis and immunotherapy of human type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1989 Oct;32(10):703–708. doi: 10.1007/BF00274528. [DOI] [PubMed] [Google Scholar]
- Langholz E., Brynskov J., Bendtzen K., Vilien M., Binder V. Treatment of Crohn's disease with fusidic acid: an antibiotic with immunosuppressive properties similar to cyclosporin. Aliment Pharmacol Ther. 1992 Aug;6(4):495–502. doi: 10.1111/j.1365-2036.1992.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Langholz E., Brynskov J., Freund L. G., Bendtzen K. Fusidic acid for Behçet's colitis: a novel approach to T-cell specific immunosuppressive therapy. Dan Med Bull. 1991 Jun;38(3):284–284. [PubMed] [Google Scholar]
- Mandrup-Poulsen T., Helqvist S., Mølvig J., Wogensen L. D., Nerup J. Cytokines as immune effector molecules in autoimmune endocrine diseases with special reference to insulin-dependent diabetes mellitus. Autoimmunity. 1989;4(3):191–234. doi: 10.3109/08916938909003049. [DOI] [PubMed] [Google Scholar]
- Miethke T., Wahl C., Heeg K., Echtenacher B., Krammer P. H., Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992 Jan 1;175(1):91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa J., Yamamoto K., Hanafusa T., Itoh N., Nakagawa C., Otsuka A., Katsura H., Yamagata K., Miyazaki A., Kono N. Preventive effect of a new immunosuppressant FK-506 on insulitis and diabetes in non-obese diabetic mice. Diabetologia. 1990 Aug;33(8):503–505. doi: 10.1007/BF00405113. [DOI] [PubMed] [Google Scholar]
- Natanson C., Hoffman W. D., Suffredini A. F., Eichacker P. Q., Danner R. L. Selected treatment strategies for septic shock based on proposed mechanisms of pathogenesis. Ann Intern Med. 1994 May 1;120(9):771–783. doi: 10.7326/0003-4819-120-9-199405010-00009. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Borghi M. O., Meroni P. L., Barcellini W., Fain C., Di Marco R., Menta R., Schorlemmer H. U., Bruno G., Magro G. Prevention of cyclophosphamide-induced diabetes in the NOD/WEHI mouse with deoxyspergualin. Clin Exp Immunol. 1993 Feb;91(2):232–236. doi: 10.1111/j.1365-2249.1993.tb05888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Di Marco R., Morrone S., Zaccone P., Lembo D., Grasso S., Santoni A., Meroni P. L., Bendtzen K. Reduction of spontaneous autoimmune diabetes in diabetes-prone BB rats with the novel immunosuppressant fusidic acid. Effect on T-cell proliferation and production of interferon-gamma. Immunology. 1994 Feb;81(2):317–321. [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Meroni P. L., Di Marco R., Grasso S., Barcellini W., Borghi M. O., Lunetta M., Mughini L., Menta R., Schorlemmer H. U. The effects of deoxyspergualin on the development of diabetes in diabetes-prone BB rats. Scand J Immunol. 1992 Sep;36(3):415–420. doi: 10.1111/j.1365-3083.1992.tb02955.x. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Meroni P. L., Lunetta M., Vigo R., Palermo T., Papalia D., Barcellini W., Di Mauro M., Caruso-Nicoletti M., Mughini L. Sodium fusidate and increased remission rate of insulin-dependent diabetes mellitus. Lancet. 1991 May 25;337(8752):1292–1292. doi: 10.1016/0140-6736(91)92964-4. [DOI] [PubMed] [Google Scholar]
- Reeves D. S. The pharmacokinetics of fusidic acid. J Antimicrob Chemother. 1987 Oct;20(4):467–476. doi: 10.1093/jac/20.4.467. [DOI] [PubMed] [Google Scholar]
- Sprung C. L., Caralis P. V., Marcial E. H., Pierce M., Gelbard M. A., Long W. M., Duncan R. C., Tendler M. D., Karpf M. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med. 1984 Nov 1;311(18):1137–1143. doi: 10.1056/NEJM198411013111801. [DOI] [PubMed] [Google Scholar]
- Strandell E., Sandler S. Protection against hyperglycemia in female nonobese diabetic mice treated with 15-deoxyspergualin. Autoimmunity. 1992;14(2):159–165. doi: 10.3109/08916939209083136. [DOI] [PubMed] [Google Scholar]
- Thomson A. W. The spectrum of action of new immunosuppressive drugs. Clin Exp Immunol. 1992 Aug;89(2):170–173. doi: 10.1111/j.1365-2249.1992.tb06927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Yasunami R., Bach J. F. Anti-suppressor effect of cyclophosphamide on the development of spontaneous diabetes in NOD mice. Eur J Immunol. 1988 Mar;18(3):481–484. doi: 10.1002/eji.1830180325. [DOI] [PubMed] [Google Scholar]
- von Daehne W., Godtfredsen W. O., Rasmussen P. R. Structure-activity relationships in fusidic acid-type antibiotics. Adv Appl Microbiol. 1979;25:95–146. doi: 10.1016/s0065-2164(08)70148-5. [DOI] [PubMed] [Google Scholar]



