Abstract
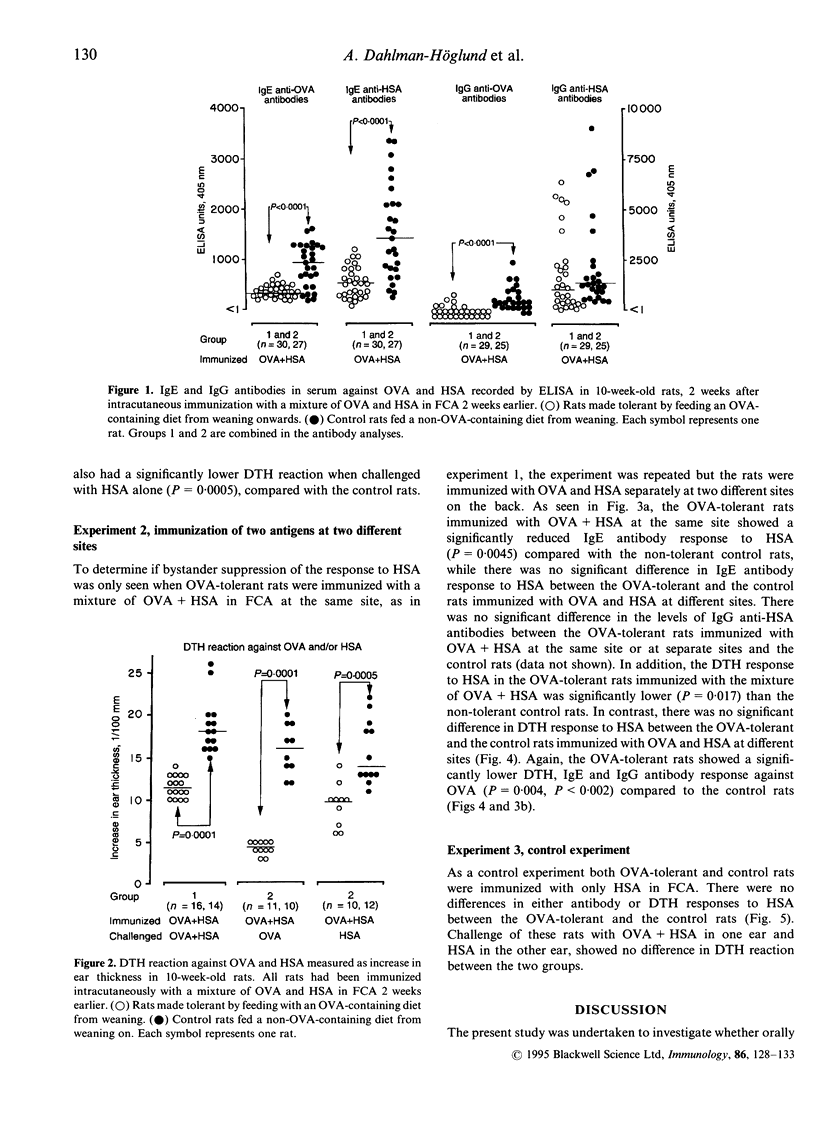
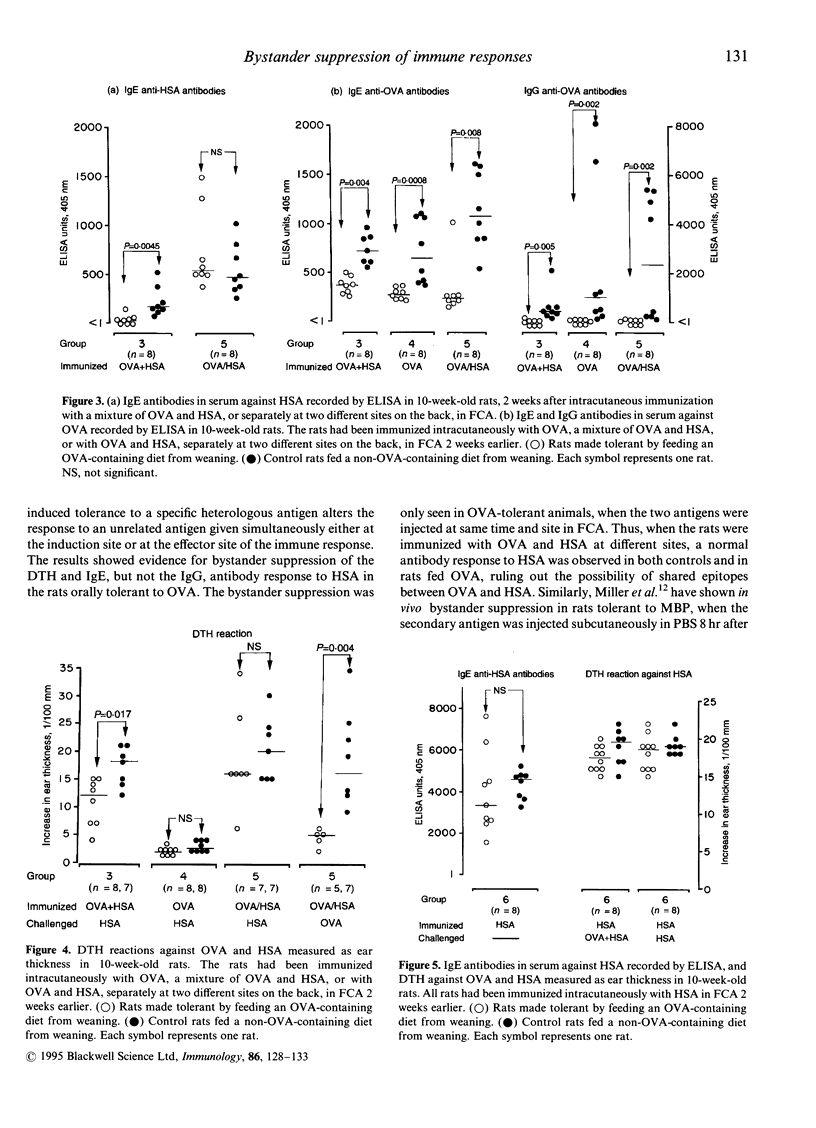
Bystander suppression of delayed-type hypersensitivity (DTH) and the antibody response to human serum albumin (HSA) were studied in young normal rats and in young rats made partially tolerant to ovalbumin (OVA) by feeding an OVA-containing diet for 4 weeks from weaning. At 2 months of age, the animals were intracutaneously immunized with a mixture of OVA and HSA in Freund's complete adjuvant (FCA) at one site of the back, or separately at two different sites on the back. All rats made orally tolerant to OVA showed a significantly reduced IgE and IgG anti-OVA antibody production and DTH response to OVA, compared to the controls. OVA-fed rats subsequently immunized with a mixture of OVA + HSA had significantly lower IgE and DTH responses to HSA than the controls. When rats were immunized with OVA and HSA at two different sites, however, there was no difference in the response to HSA between the OVA-fed rats and the control rats, which rules out the possibility of shared epitopes between the antigens. Ear-challenge with the mixture of OVA + HSA gave a significantly lower DTH reaction in the tolerant rats immunized with a mixture of the antigens, compared to the control rats. However, suppression of the DTH reaction was not seen when tolerant and control rats were immunized with HSA alone and challenged with the mixture of OVA + HSA in one ear. These results present evidence that young rats orally tolerant to one antigen show a suppressed T-cell and antibody response to an unrelated antigen, provided that the two antigens are given in a mixture during the inductive phase. There was no evidence for bystander suppression of the T-cell response at the effector site.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burstein H. J., Abbas A. K. In vivo role of interleukin 4 in T cell tolerance induced by aqueous protein antigen. J Exp Med. 1993 Feb 1;177(2):457–463. doi: 10.1084/jem.177.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A. How B and T cells talk to each other. Nature. 1994 Feb 3;367(6462):425–428. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- Dahlman A., Ahlstedt S., Hanson L. A., Telemo E., Wold A. E., Dahlgren U. I. Induction of IgE antibodies and T-cell reactivity to ovalbumin in rats colonized with Escherichia coli genetically manipulated to produce ovalbumin. Immunology. 1992 Jun;76(2):225–228. [PMC free article] [PubMed] [Google Scholar]
- Ferguson T. A., Peters T., Jr, Reed R., Pesce A. J., Michael J. G. Immunoregulatory properties of antigenic fragments from bovine serum albumin. Cell Immunol. 1983 May;78(1):1–12. doi: 10.1016/0008-8749(83)90254-x. [DOI] [PubMed] [Google Scholar]
- Friedman A., Weiner H. L. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6688–6692. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lider O., Santos L. M., Lee C. S., Higgins P. J., Weiner H. L. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. II. Suppression of disease and in vitro immune responses is mediated by antigen-specific CD8+ T lymphocytes. J Immunol. 1989 Feb 1;142(3):748–752. [PubMed] [Google Scholar]
- Mayrhofer G., Pugh C. W., Barclay A. N. The distribution, ontogeny and origin in the rat of Ia-positive cells with dendritic morphology and of Ia antigen in epithelia, with special reference to the intestine. Eur J Immunol. 1983 Feb;13(2):112–122. doi: 10.1002/eji.1830130206. [DOI] [PubMed] [Google Scholar]
- McMenamin C., Holt P. G. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but MHC class II-restricted CD4+ T cell-dependent immune deviation resulting in selective suppression of immunoglobulin E production. J Exp Med. 1993 Sep 1;178(3):889–899. doi: 10.1084/jem.178.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed D., Friedman A. Direct evidence for anergy in T lymphocytes tolerized by oral administration of ovalbumin. Eur J Immunol. 1993 Apr;23(4):935–942. doi: 10.1002/eji.1830230426. [DOI] [PubMed] [Google Scholar]
- Miller A., Lider O., Abramsky O., Weiner H. L. Orally administered myelin basic protein in neonates primes for immune responses and enhances experimental autoimmune encephalomyelitis in adult animals. Eur J Immunol. 1994 May;24(5):1026–1032. doi: 10.1002/eji.1830240503. [DOI] [PubMed] [Google Scholar]
- Miller A., Lider O., Roberts A. B., Sporn M. B., Weiner H. L. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Lider O., Weiner H. L. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med. 1991 Oct 1;174(4):791–798. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Zhang Z. J., Sobel R. A., al-Sabbagh A., Weiner H. L. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. VI. Suppression of adoptively transferred disease and differential effects of oral vs. intravenous tolerization. J Neuroimmunol. 1993 Jul;46(1-2):73–82. doi: 10.1016/0165-5728(93)90235-q. [DOI] [PubMed] [Google Scholar]
- Murphy D. B. T cell mediated immunosuppression. Curr Opin Immunol. 1993 Jun;5(3):411–417. doi: 10.1016/0952-7915(93)90061-v. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. Down-regulation of immune responses to inhaled antigen: studies on the mechanism of induced suppression. Immunology. 1985 Dec;56(4):635–642. [PMC free article] [PubMed] [Google Scholar]
- Strobel S., Ferguson A. Immune responses to fed protein antigens in mice. 3. Systemic tolerance or priming is related to age at which antigen is first encountered. Pediatr Res. 1984 Jul;18(7):588–594. doi: 10.1203/00006450-198407000-00004. [DOI] [PubMed] [Google Scholar]
- Strobel S., Ferguson A. Modulation of intestinal and systemic immune responses to a fed protein antigen, in mice. Gut. 1986 Jul;27(7):829–837. doi: 10.1136/gut.27.7.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi T. B., Jr Oral tolerance. Transplantation. 1980 May;29(5):353–356. doi: 10.1097/00007890-198005000-00001. [DOI] [PubMed] [Google Scholar]
- Whitacre C. C., Gienapp I. E., Orosz C. G., Bitar D. M. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J Immunol. 1991 Oct 1;147(7):2155–2163. [PubMed] [Google Scholar]
- Wold A. E., Dahlgren U. I., Ahlstedt S., Hanson L. A. Rats are sensitive to induction of tolerance by feeding only at young age. Monogr Allergy. 1988;24:251–252. [PubMed] [Google Scholar]
- Wold A. E., Dahlgren U. I., Hanson L. A., Mattsby-Baltzer I., Midvetdt T. Difference between bacterial and food antigens in mucosal immunogenicity. Infect Immun. 1989 Sep;57(9):2666–2673. doi: 10.1128/iai.57.9.2666-2673.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


