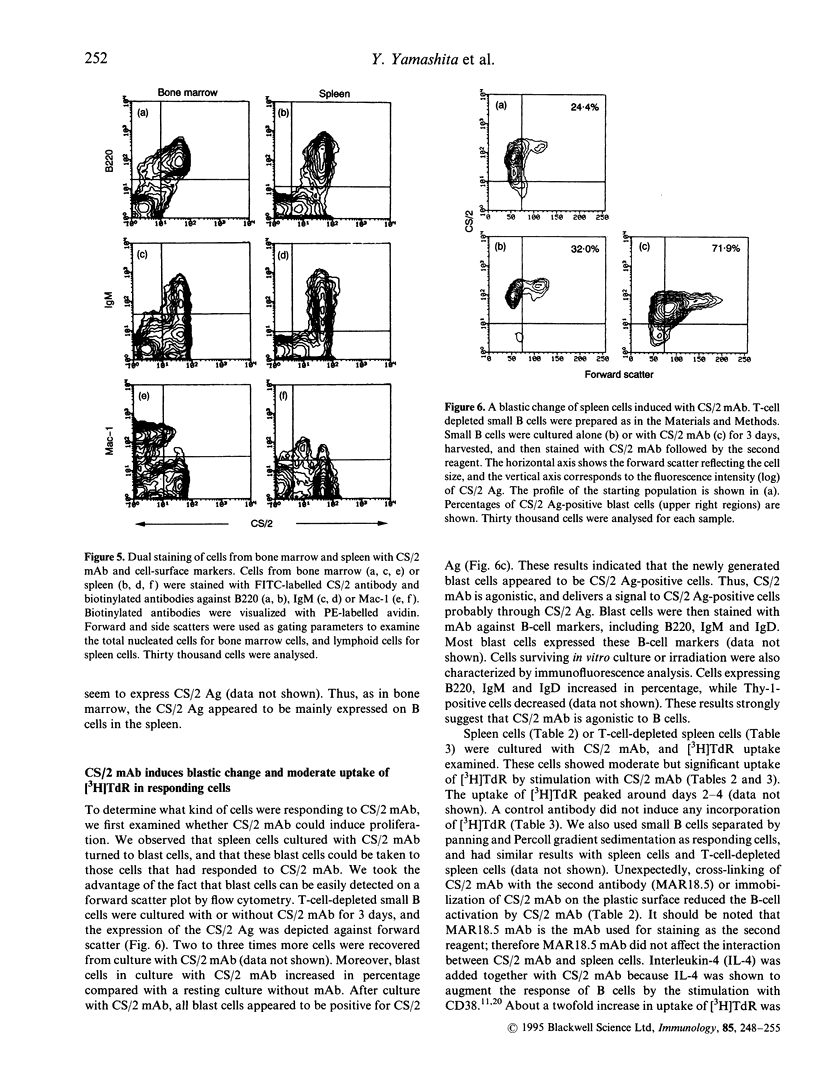
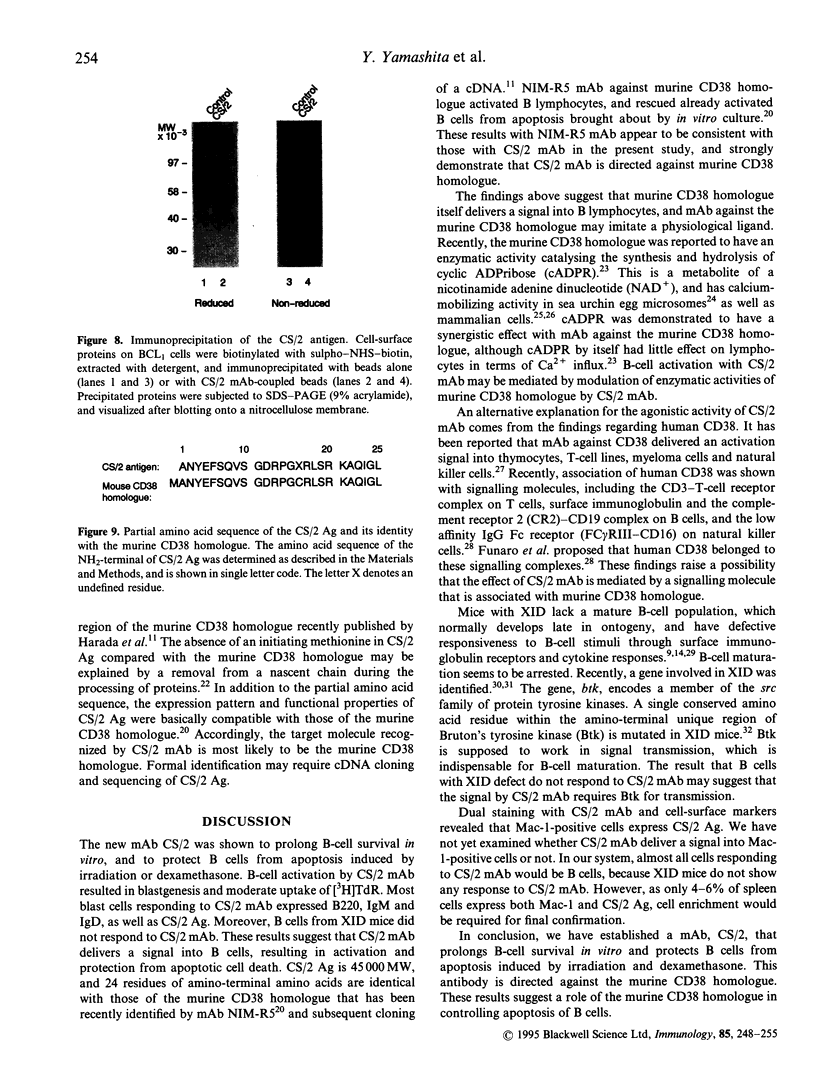
Abstract
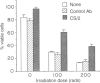
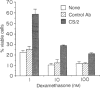
A novel monoclonal antibody (mAb) was established in an attempt to look for a cell-surface molecule that delivered a signal regulating apoptotic cell death of B cells. Because spleen cells in resting culture die from apoptosis, mAb were looked for that were able to prolong spleen cell survival in vitro. This screening selected mAb CS/2. CS/2 not only prolonged spleen cell survival in vitro, but also protected spleen cells from apoptotic cell death brought about by irradiation or dexamethasone. Moreover, stimulation of spleen cells with CS/2 mAb induced changes of cells to blastoid morphology, and a significant uptake of [3H]thymidine ([3H]TdR). The antigen recognized by CS/2 mAb (CS/2 Ag) was expressed on preB cells, B cells, and Mac-1+ cells. The cells surviving in vitro culture or irradiation in response to ligation with CS/2 mAb were mostly B cells expressing the CS/2 Ag. In addition, B cells from X-linked immunodeficient (XID) mice did not respond to CS/2 mAb. These results indicate that CS/2 mAb is agonistic to B cells, and that XID mice are deficient in this CS/2 mAb-mediated activation pathway. Determination of the amino-terminal 24 amino acid residues revealed that the CS/2 Ag appears to be identical to the CS/2 mAb is directed against a murine CD38 homologue, and suggest a possible role of the murine CD38 homologue in controlling apoptotic cell death of B cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bishop G. A., Haughton G. Induced differentiation of a transformed clone of Ly-1+ B cells by clonal T cells and antigen. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7410–7414. doi: 10.1073/pnas.83.19.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper D. L., Walseth T. F., Dargie P. J., Lee H. C. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J Biol Chem. 1987 Jul 15;262(20):9561–9568. [PubMed] [Google Scholar]
- Coffman R. L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Funaro A., De Monte L. B., Dianzani U., Forni M., Malavasi F. Human CD38 is associated to distinct molecules which mediate transmembrane signaling in different lineages. Eur J Immunol. 1993 Oct;23(10):2407–2411. doi: 10.1002/eji.1830231005. [DOI] [PubMed] [Google Scholar]
- Funaro A., Spagnoli G. C., Ausiello C. M., Alessio M., Roggero S., Delia D., Zaccolo M., Malavasi F. Involvement of the multilineage CD38 molecule in a unique pathway of cell activation and proliferation. J Immunol. 1990 Oct 15;145(8):2390–2396. [PubMed] [Google Scholar]
- Go N. F., Castle B. E., Barrett R., Kastelein R., Dang W., Mosmann T. R., Moore K. W., Howard M. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990 Dec 1;172(6):1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow C. C. Transgenic mice and analysis of B-cell tolerance. Annu Rev Immunol. 1992;10:489–518. doi: 10.1146/annurev.iy.10.040192.002421. [DOI] [PubMed] [Google Scholar]
- Harada N., Santos-Argumedo L., Chang R., Grimaldi J. C., Lund F. E., Brannan C. I., Copeland N. G., Jenkins N. A., Heath A. W., Parkhouse R. M. Expression cloning of a cDNA encoding a novel murine B cell activation marker. Homology to human CD38. J Immunol. 1993 Sep 15;151(6):3111–3118. [PubMed] [Google Scholar]
- Hartley S. B., Cooke M. P., Fulcher D. A., Harris A. W., Cory S., Basten A., Goodnow C. C. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993 Feb 12;72(3):325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Hirel P. H., Schmitter M. J., Dessen P., Fayat G., Blanquet S. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8247–8251. doi: 10.1073/pnas.86.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoshi Y., Okada Y., Sonoda E., Tominaga A., Makino M., Suzuki K., Kinoshita J., Komuro K., Mizuochi T., Takatsu K. Delayed progression of a murine retrovirus-induced acquired immunodeficiency syndrome in X-linked immunodeficient mice. J Exp Med. 1993 Mar 1;177(3):621–626. doi: 10.1084/jem.177.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoshi Y., Sonoda E., Kikuchi Y., Yonehara S., Nakauchi H., Takatsu K. IL-5 receptor positive B cells, but not eosinophils, are functionally and numerically influenced in mice carrying the X-linked immune defect. Int Immunol. 1993 Sep;5(9):1183–1190. doi: 10.1093/intimm/5.9.1183. [DOI] [PubMed] [Google Scholar]
- Howard M., Grimaldi J. C., Bazan J. F., Lund F. E., Santos-Argumedo L., Parkhouse R. M., Walseth T. F., Lee H. C. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993 Nov 12;262(5136):1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- Jackson D. G., Bell J. I. Isolation of a cDNA encoding the human CD38 (T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J Immunol. 1990 Apr 1;144(7):2811–2815. [PubMed] [Google Scholar]
- Koshiyama H., Lee H. C., Tashjian A. H., Jr Novel mechanism of intracellular calcium release in pituitary cells. J Biol Chem. 1991 Sep 15;266(26):16985–16988. [PubMed] [Google Scholar]
- Krolick K. A., Isakson P. C., Uhr J. W., Vitetta E. S. BCL1, a murine model for chronic lymphocytic leukemia: use of the surface immunoglobulin idiotype for the detection and treatment of tumor. Immunol Rev. 1979;48:81–106. doi: 10.1111/j.1600-065x.1979.tb00299.x. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Joshua D. E., Williams G. T., Smith C. A., Gordon J., MacLennan I. C. Mechanism of antigen-driven selection in germinal centres. Nature. 1989 Dec 21;342(6252):929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Miyake K., Hasunuma Y., Yagita H., Kimoto M. Requirement for VLA-4 and VLA-5 integrins in lymphoma cells binding to and migration beneath stromal cells in culture. J Cell Biol. 1992 Nov;119(3):653–662. doi: 10.1083/jcb.119.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Yamashita Y., Hitoshi Y., Takatsu K., Kimoto M. Murine B cell proliferation and protection from apoptosis with an antibody against a 105-kD molecule: unresponsiveness of X-linked immunodeficient B cells. J Exp Med. 1994 Oct 1;180(4):1217–1224. doi: 10.1084/jem.180.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemazee D. A., Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989 Feb 9;337(6207):562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Negative selection of lymphocytes. Cell. 1994 Jan 28;76(2):229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Perandones C. E., Illera V. A., Peckham D., Stunz L. L., Ashman R. F. Regulation of apoptosis in vitro in mature murine spleen T cells. J Immunol. 1993 Oct 1;151(7):3521–3529. [PubMed] [Google Scholar]
- Rawlings D. J., Saffran D. C., Tsukada S., Largaespada D. A., Grimaldi J. C., Cohen L., Mohr R. N., Bazan J. F., Howard M., Copeland N. G. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993 Jul 16;261(5119):358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tarduchy G., Collins M., López-Rivas A. Regulation of apoptosis in interleukin-3-dependent hemopoietic cells by interleukin-3 and calcium ionophores. EMBO J. 1990 Sep;9(9):2997–3002. doi: 10.1002/j.1460-2075.1990.tb07492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Argumedo L., Teixeira C., Preece G., Kirkham P. A., Parkhouse R. M. A B lymphocyte surface molecule mediating activation and protection from apoptosis via calcium channels. J Immunol. 1993 Sep 15;151(6):3119–3130. [PubMed] [Google Scholar]
- Scher I. CBA/N immune defective mice; evidence for the failure of a B cell subpopulation to be expressed. Immunol Rev. 1982;64:117–136. doi: 10.1111/j.1600-065x.1982.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Takasawa S., Nata K., Yonekura H., Okamoto H. Cyclic ADP-ribose in insulin secretion from pancreatic beta cells. Science. 1993 Jan 15;259(5093):370–373. doi: 10.1126/science.8420005. [DOI] [PubMed] [Google Scholar]
- Tsukada S., Saffran D. C., Rawlings D. J., Parolini O., Allen R. C., Klisak I., Sparkes R. S., Kubagawa H., Mohandas T., Quan S. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993 Jan 29;72(2):279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- Vetrie D., Vorechovský I., Sideras P., Holland J., Davies A., Flinter F., Hammarström L., Kinnon C., Levinsky R., Bobrow M. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993 Jan 21;361(6409):226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Positive selection of lymphocytes. Cell. 1994 Jan 28;76(2):219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]